绣线菊苷 | 618-65-5
中文名称
绣线菊苷
中文别名
水杨醛葡糖甙水合物;水杨醛-β-D-葡糖苷;柳醛苷;水杨醛葡糖甙
英文名称
helicin
英文别名
2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxybenzaldehyde
CAS
618-65-5
化学式
C13H16O7
mdl
——
分子量
284.266
InChiKey
BGOFCVIGEYGEOF-UJPOAAIJSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:178 °C(lit.)
-
比旋光度:D20 -60° (c = 1.4)
-
沸点:386.76°C (rough estimate)
-
密度:1.3514 (rough estimate)
-
溶解度:溶于甲醇、水
-
LogP:-1.440 (est)
-
碰撞截面:161.4 Ų [M+Na]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):-1.5
-
重原子数:20
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.46
-
拓扑面积:116
-
氢给体数:4
-
氢受体数:7
安全信息
-
危险品标志:Xi
-
安全说明:S24/25,S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2932999099
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲酰基苯基 2,3,4,6-四-O-乙酰基-beta-D-吡喃葡萄糖苷 helicin acetate 14581-83-0 C21H24O11 452.415 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲酰基苯基 2,3,4,6-四-O-乙酰基-beta-D-吡喃葡萄糖苷 helicin acetate 14581-83-0 C21H24O11 452.415 草木樨苷 melilotoside 618-67-7 C15H18O8 326.303 —— Methyl o-coumarate-β-D-glucoside 148225-41-6 C16H20O8 340.33
反应信息
-
作为反应物:描述:绣线菊苷 在 三氧化硫吡啶 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 12.0h, 以39%的产率得到2-formylphenyl β-D-glucopyranoside-6-sulfate参考文献:名称:海藻糖6-磷酸磷酸酶可逆抑制剂的合理设计摘要:在某些生物中,环境压力触发了海藻糖生物合成,海藻糖生物合成由海藻糖6-磷酸合酶和海藻糖6-磷酸磷酸酶(T6PP)共同催化。T6PP催化将海藻糖6-磷酸酯(T6P)水解为海藻糖和无机磷酸酯,是开发抗菌,抗真菌和抗蠕虫药的有希望的目标。在这里,我们报告设计,合成和评估的芳基d-吡喃葡萄糖苷6硫酸盐的库,以用作小分子T6PP抑制剂的原型。稳态动力学技术用于测量一组源自病原体马来亚布鲁氏菌(Brugia malayi),猪scar虫(Ascaris suum),多种结构上不同的T6PP直系同源物的抑制常数(K i)。结核分枝杆菌,博伊氏志贺氏菌和鼠伤寒沙门氏菌。发现这些T6PP直系同源物中最活跃的抑制剂4-正辛基苯基α - d-吡喃葡萄糖苷6-硫酸盐(9a)的结合亲和力在低微摩尔范围内。与马来西亚芽孢杆菌T6PP直系同源物的9a的K i为5.3±0.6μM,比底物Michaelis常数小70倍。9DOI:10.1016/j.ejmech.2017.02.001
-
作为产物:描述:2-(2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyloxy)benzaldehyde 在 potassium carbonate 作用下, 以 甲醇 、 水 为溶剂, 反应 0.17h, 以92%的产率得到绣线菊苷参考文献:名称:相转移催化的d-葡萄糖基化:苯甲酰化的芳基β-d-吡喃葡萄糖苷和β-d-吡喃葡萄糖基取代的肉桂酸酯的合成摘要:摘要用2,3,4,6-四-O-苯甲酰基-α-吡喃葡萄糖基溴化物进行相转移催化的螯合酚醛糖苷和取代的肉桂酸的d-葡萄糖基化反应,导致苯甲酰化的芳基β-吡喃葡萄糖苷和分别以良好的吡喃糖苷类取代-β-d-葡萄糖基吡喃糖基肉桂酸酯和分别以良好的产率分别形成取代的-β-d-葡萄糖基吡喃糖基肉桂酸酯。结果最终证实了较早的报道,即相转移方法需要碳水化合物部分中C-2或C-6处的非参与基团。在所有这些反应中,得到次要的副产物1,2,3,4,6-Penta-O-苯甲酰基-β-d-吡喃葡萄糖。讨论了相转移方法的可能机理。DOI:10.1016/0008-6215(87)80206-9
-
作为试剂:描述:1-amino-2,3-bis-benzoyloxy-propane 在 sodium acetate 、 溶剂黄146 、 绣线菊苷 作用下, 生成 (-)-1-amino-2,3-bis-benzoyloxy-propane参考文献:名称:Bergmann, Hoppe-Seyler's Zeitschrift fur Physiologische Chemie, 1924, vol. 137, p. 42摘要:DOI:
文献信息
-
Four Orders of Magnitude Rate Increase in Artificial Enzyme-Catalyzed Aryl Glycoside Hydrolysis作者:Fernando Ortega-Caballero、Jeannette Bjerre、Line Skall Laustsen、Mikael BolsDOI:10.1021/jo050861w日期:2005.9.1kcat/kuncat ranging from 4 to 7100. Hydrolysis of a phenyl β-d-glucoside or the thioglycoside tolylthio β-d-glucoside was also catalyzed. From a series of prepared analogues of 1 it was found that the catalysis was associated with the hydroxyl groups α to the nitril groups. The monocyanohydrin 6-C-cyano-β-cyclodextrin (3) was also found to catalyze the hydrolysis of 4-nitrophenyl β-glucopyranoside with(6 A R,6 D R)-6 A,6 D -Di- C-氰基-β-环糊精(1)和6 A,6 D -di- C-氰基-α-环糊精(2)合成并结果显示,其遵循米利斯-门腾动力学反应,可催化芳基糖苷水解为葡萄糖和苯酚。在pH8.0和4-硝基苯基α-D-吡喃葡萄糖苷的59℃水解物通过催化1与ķ中号= 10.5±1.5毫米,ķ猫= 1.42(±0.09)×10 - 4个小号- 1,和ķ猫/ ķ uncat = 7922.用的浓度观察到催化1低至10μM。包含糖部分和4-硝基-立体化学的变化的其他芳基糖苷的水解,2-硝基,2- aldehydo-,和2,4-二硝基还通过催化1和2与ķ猫/ ķ uncat范围为4至7100。还催化了苯基β- d-葡糖苷或硫代糖苷甲苯基硫代β- d-葡糖苷的水解。来自一系列准备好的类似物1发现该催化作用与腈基上的羟基α有关。所述monocyanohydrin 6- Ç氰
-
MOF-derived cobalt nanoparticles catalyze a general synthesis of amines作者:Rajenahally V. Jagadeesh、Kathiravan Murugesan、Ahmad S. Alshammari、Helfried Neumann、Marga-Martina Pohl、Jörg Radnik、Matthias BellerDOI:10.1126/science.aan6245日期:2017.10.20preparation entailed template assembly of cobalt-diamine-dicarboxylic acid metal organic frameworks on carbon and subsequent pyrolysis under inert atmosphere. The resulting stable and reusable catalysts were active for synthesis of primary, secondary, tertiary, and N-methylamines (more than 140 examples). The reaction couples easily accessible carbonyl compounds (aldehydes and ketones) with ammonia, aminesMOF 为制造胺奠定了基础 还原胺化是化学家用来制造碳氮键的常用方法。该反应通常需要贵金属催化剂,将氨或其他胺与羰基化合物偶联,然后与氢气偶联。贾加迪什等人。报告了一类非贵重的钴纳米粒子,可在非常广泛的底物上催化这种反应,包括具有药用价值的复杂分子(参见 Chen 和 Xu 的观点)。钴首先嵌入金属有机框架 (MOF) 中,加热后转变为石墨壳。催化剂可以方便地从产品中分离出来,最多可循环使用六次。科学,这个问题 p。326; 另见第。304 由金属-有机骨架前体制备的钴纳米颗粒可催化非常广泛的还原胺化反应。用于合成药物相关化合物的贱金属催化剂的开发仍然是化学研究的一个重要目标。在这里,我们报告说,由石墨壳包裹的钴纳米颗粒是广泛有效的还原胺化催化剂。它们方便实用的制备需要在碳上模板组装钴-二胺-二羧酸金属有机骨架,然后在惰性气氛下热解。所得稳定且可重复使用的催化剂对于合成伯胺、仲胺、叔胺和 N-甲胺(超过
-
Highly Selective Primary Alkoxycarboxylation and Esterification of Unprotected Pyranose Derivatives Mediated by Scandium(III) Triflate Catalysis作者:Michael S. McClure、Malcolm B. Berry、Darren Caine、Claire Crawford、Brian C. Crump、Bobby N. Glover、Sandeep B. Kedia、Alan Millar、Mark B. Mitchell、Christopher J. Nichols、Daniel E. Patterson、Jeremiah PowersDOI:10.1002/ejoc.201200261日期:2012.7A highly selective method for the alkoxycarboxylation and acylation of primary alcohols of pyranose derivatives is described. The reaction is high yielding and proceeds under mild conditions with 0.15–1 mol-% Sc(OTf)3 used in combination with anhydrides or pyrocarbonates at 40–50 °C. Selectivities observed for alkoxycarboxylation of unprotected pyranose derivatives are > 95 %, and this constitutes
-
Synthesis of glycosylated cationic porphyrins as potential agents in photodynamic therapy作者:Khalid Driaf、Robert Granet、Pierre krausz、Mourad Kaouadji、Bernard Verneuil、François Thomasson、Albert José Chulia、Marenglen Spiro、Jean-Claude Biais、Gérard BolbachDOI:10.1139/v96-172日期:1996.8.1
Trisalkylpyridinium porphyrins substituted by one glycosyl (glucosyl, maltosyl, and lactosyl) moiety have been prepared in acceptable yields. These glycosylated cationic porphyrins have been synthesized from pyrrole condensed with 4-pyridinecarboxaldehyde, and suitable ortho- or para peracetylglycosyloxybenzaldehyde derivatives in refluxing propionic acid –Ac2O followed by action of alkyliodide in DMF. Deprotection of the glycosylated moieties led to a new class of representative glycosylated porphyrins. Key words: porphyrins, cationic, glycosylated; phototherapy, cancer.
-
Glycosylated tetrahydrosalens as multifunctional molecules for Alzheimer's therapy作者:Tim Storr、Lauren E. Scott、Meryn L. Bowen、David E. Green、Katherine H. Thompson、Harvey J. Schugar、Chris OrvigDOI:10.1039/b902545f日期:——The tetrahydrosalens N,N′-bis(2-hydroxybenzyl)-ethane-1,2-diamine (H2L1), N,N′-bis(2-hydroxybenzyl)-(−)-1,2-cyclohexane-(1R,2R)-diamine (H2L2), N,N′-bis(2-hydroxybenzyl)-N,N′-dimethyl-ethane-1,2-diamine (H2L3), N,N′-bis(2-hydroxybenzyl)-N,N′-dibenzyl-ethane-1,2-diamine (H2L4), and N,N′-bis(2-(4-tert-butyl)hydroxybenzyl)-ethane-1,2-diamine (H2L5), as well as their prodrug glycosylated forms, GL1–5, have been prepared and evaluated in vitro for their potential use as Alzheimer's disease (AD) therapeutics. Dysfunctional interactions of metal ions, especially those of Cu, Zn, and Fe, with the amyloid-β (Aβ) peptide are hypothesised to play an important role in the aetiology of AD, and disruption of these aberrant metal–peptide interactions via chelation therapy holds considerable promise as a therapeutic strategy. Tetrahydrosalens such as H2L1–5 have a significant affinity for metal ions, and thus should be able to compete with the Aβ peptide for Cu, Zn, and Fe in the brain. This activity was assayed in vitrovia a turbidity assay; H2L1 and H2L3 were found to attenuate Aβ1–40 aggregation after exposure to Cu2+ and Zn2+. In addition, H2L1–5 were determined to be potent antioxidants on the basis of an in vitro antioxidant assay. GL1–5 were prepared as metal binding prodrugs; glycosylation is intended to prevent systemic metal binding, improve solubility, and enhance brain uptake. Enzymatic (β-glucosidase) deprotection of the carbohydrate moieties was facile, with the exception of GL4, demonstrating the general feasibility of this prodrug approach. Finally, a representative prodrug, GL3, was determined to be non-toxic over a large concentration range in a cell viability assay.四氢萨林(tetrahydrosalens)N,N′-双(2-羟基苯基)-乙烷-1,2-二胺(H2L1)、N,N′-双(2-羟基苯基)-(−)-1,2-环己烷-(1R,2R)-二胺(H2L2)、N,N′-双(2-羟基苯基)-N,N′-二甲基-乙烷-1,2-二胺(H2L3)、N,N′-双(2-羟基苯基)-N,N′-二苄基-乙烷-1,2-二胺(H2L4)以及N,N′-双(2-(4-叔丁基)羟基苯基)-乙烷-1,2-二胺(H2L5),连同它们的前药糖苷化形式GL1–5,已被合成并在体外评估其作为阿尔茨海默病(AD)治疗剂的潜力。金属离子(特别是铜、锌和铁)与淀粉样β(Aβ)肽的功能失调相互作用被假设在阿尔茨海默病的病因中发挥重要作用,通过螯合疗法破坏这些异常的金属–肽相互作用被认为是一种极具前景的治疗策略。像H2L1–5这样的四氢萨林对金属离子有显著亲和力,因此应该能够与Aβ肽竞争大脑中的铜、锌和铁。通过浊度测定法在体外检测这种活性;H2L1和H2L3在Cu2+和Zn2+的存在下被发现能够减弱Aβ1–40的聚集。此外,根据体外抗氧化剂测定,H2L1–5被确定为有效的抗氧化剂。GL1–5作为金属结合的前药制备;糖苷化旨在防止系统性金属结合,提高溶解度并增强大脑摄取。除了GL4以外,碳水化合物部分的酶(β-葡萄糖苷酶)去保护过程相对容易,证明了这一前药方法的一般可行性。最后,一种代表性前药GL3在细胞活性测定中被确定在较大浓度范围内是非毒性的。
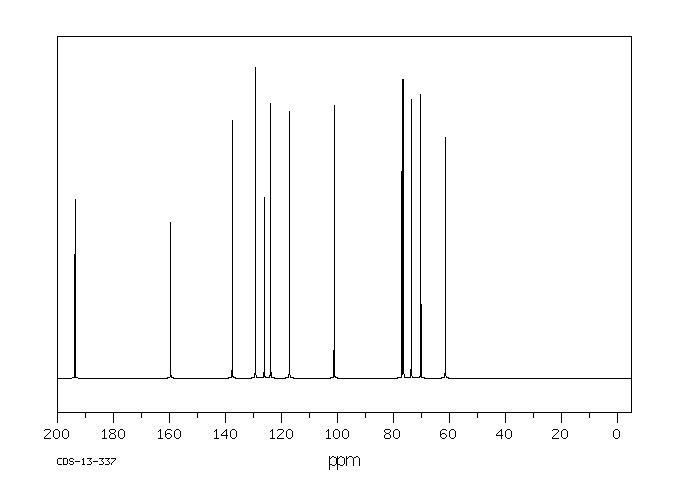
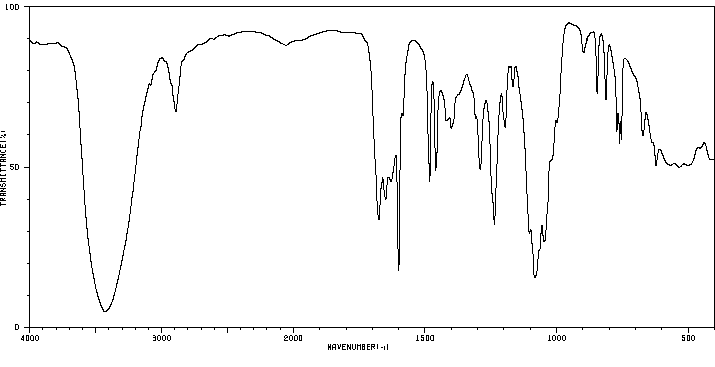
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷