7-hydroxy-3-(3-hydroxy-2,4-dimethoxyphenyl)chromen-4-one
中文名称
——
中文别名
——
英文名称
7-hydroxy-3-(3-hydroxy-2,4-dimethoxyphenyl)chromen-4-one
英文别名
4',7-dihydroxy-3',5'-dimethoxyisoflavone;7,4′‐dihydroxy‐3′,5′‐dimethoxyisoflavone;7-hydroxy-3-(4-hydroxy-3,5-dimethoxyphenyl)chromen-4-one
CAS
——
化学式
C17H14O6
mdl
——
分子量
314.295
InChiKey
KAAIDCFNSXASKK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:23
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:85.2
-
氢给体数:2
-
氢受体数:6
反应信息
-
作为产物:参考文献:名称:橄榄黄异黄酮类似物的合成及生物学评价摘要:通过涉及有机铅试剂的配体偶联反应,制备了从Dalbergia oliveri Gamble分离出的Mucronulatol 1和violanone 2,以及相应的异黄酮3。生物学研究表明,仅对于异黄烷1,其对HBL100白血病细胞系具有明显的细胞毒性作用,IC 50值为5.7μM 。所有药物均会适度地抑制体外微管的组装,而与细胞毒性能力无关。天然化合物1和2没有抗菌活性,但是是尖孢镰刀菌植物致病性真菌生长的有效抑制剂。DOI:10.1016/j.tet.2007.10.030
文献信息
-
A Versatile Microbial System for Biosynthesis of Novel Polyphenols with Altered Estrogen Receptor Binding Activity作者:Joseph A. Chemler、Chin Giaw Lim、John L. Daiss、Mattheos A.G. KoffasDOI:10.1016/j.chembiol.2010.03.010日期:2010.4Isoflavonoids possess enormous potential for human health with potential impact on heart disease and cancer, and some display striking affinities for steroid receptors. Synthesized primarily by legumes, isoflavonoids are present in low and variable abundance within complex mixtures, complicating efforts to assess their clinical potential. To satisfy the need for controlled, efficient, and flexible biosynthesis of isoflavonoids, a three-enzyme system has been constructed in yeast that can convert natural and synthetic flavanones into their corresponding isoflavones in practical quantities. Based on the determination of the substrate requirements of isoflavone synthase, a series of natural and nonnatural isoflavones were prepared and their binding affinities for the human estrogen receptors (ER alpha and ER beta) were determined. Structure activity relationships are suggested based on changes to binding affinities related to small variations on the isoflavone structure.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
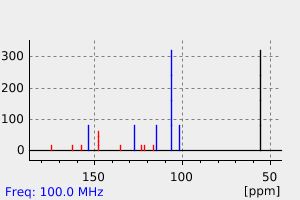
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄豆黄苷
黄豆黄素
黄豆苷元-D6
黄豆苷元-4,7-二葡糖苷
黄芪异黄烷苷,7,2'-二羟基-3',4'-二甲氧基异黄烷
黄羽扇豆魏特酮
黄细心酮 E
黄细心酮 B
鹰嘴豆芽素A
鸢尾黄酮甲素
鸢尾黄酮乙素
鸢尾黄素
鸢尾黄素
鸢尾苷
鸡豆黄素配糖物
鱼藤醇酮
鱼藤酮
鱼藤二酮
魚藤素
高紫檀素; 3,9-二甲氧基紫檀碱
高丽槐素乙酸酯
高丽槐素
顺式奥美昔芬
雌马酚
雌马酚
降香黄烃
阿比西尼亚桐素II;(6aR,11aR)-6a,11a-二氢-2,10-双(3-甲基-2-丁烯-1-基)-6H-苯并呋喃并[3,2-c][1]苯并吡喃-3,9-二醇
金雀异黄酮-D4
金雀异黄素4'-β-D-葡糖醛酸
野鹫尾苷
野鸢尾黄素
豌豆素
豆苷
西卡宁
西北甘草异黄酮
补骨脂异黄酮
补骨脂定
蟛蜞菊内酯
葛花苷
葛花宁
葛根素芹菜苷
葛根素-4'-Β-D-葡萄糖苷
葛根素
菜豆蛋白
菜豆素
菜豆异黄烷
菜豆双氢异黄酮
荧光增白剂 236
茚并[2,1-b]色烯
苯并[b]茚并[1,2-e]吡喃-6-甲醛