(E)-isopropyl cinnamate | 60512-85-8
中文名称
——
中文别名
——
英文名称
(E)-isopropyl cinnamate
英文别名
isopropyl cinnamate;isopropyl trans-cinnamate;isopropyl (E)-cinnamate;i-propyl cinnamate;propan-2-yl (E)-3-phenylprop-2-enoate
CAS
60512-85-8
化学式
C12H14O2
mdl
——
分子量
190.242
InChiKey
RGACABDFLVLVCT-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:269°C (estimate)
-
密度:1.0320
-
物理描述:Colourless viscous liquid, balsamic, sweet and dry amber type odour
-
溶解度:insoluble in water; soluble in oils
-
折光率:1.541-1.548
-
保留指数:1485
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— Methyl cinnamate 103-26-4 C10H10O2 162.188 反式-桂皮酸酐 cinnamic anhydride 21947-71-7 C18H14O3 278.307 肉桂酸 (E)-3-phenylacrylic acid 140-10-3 C9H8O2 148.161 肉桂酸 Cinnamic acid 621-82-9 C9H8O2 148.161 3-苯基丙-2-烯-1-醇 (2E)-3-phenyl-2-propen-1-ol 4407-36-7 C9H10O 134.178 反式肉桂醛 (E)-3-phenylpropenal 14371-10-9 C9H8O 132.162 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (Z)-isopropyl 3-phenylacrylate 28541-01-7 C12H14O2 190.242 反式-肉桂酸乙酯 ethyl cinnamate 4192-77-2 C11H12O2 176.215 3-苯基丙-2-烯-1-醇 (2E)-3-phenyl-2-propen-1-ol 4407-36-7 C9H10O 134.178
反应信息
-
作为反应物:描述:(E)-isopropyl cinnamate 在 sodium tetrahydroborate 作用下, 以 二乙二醇二甲醚 为溶剂, 反应 5.0h, 以2%的产率得到3-苯基丙-2-烯-1-醇参考文献:名称:Reductions of Carboxylic Acids and Esters with NaBH4 in Diglyme at 162°C摘要:Aromatic esters, including the extremely sterically hindered ester: t-amyl 2-chlorobenzoate, are readily reduced to the corresponding benzyl alcohols in high yield with NaBH4 in refluxing diglyme (162 degreesC). In sharp contrast, aliphatic esters usually gave only low yields of alcohols. Instead, diglyme fragmentation products are formed which undergo transesterification reactions, producing complex product mixtures including products such as RCOOCH2CH2OCH3. The mechanism of this process involves sodium borohydride-induced S(N)2 cleavage of diglyme (hydride attack) at high temperatures. However, when the extremely electron rich, 3,4,5-trimethoxybenzoic acid is treated with NaBH4/diglyme at 162 degreesC (with or without an equivalent of LiCl), no 3,4,5-trimethyoxybenzyl alcohol is formed. The electron rich and hindered ester, t-amyl-3,4,5-trimethoxybenzoate, also does not reduce under these conditions (with or without LiCl). However, both methyl and isopropyl 3,4,5-trimethoxybenzoate esters were converted into 3,4,5-trimethyoxybenzyl alcohol in good yields in NaBH4/diglyme/LiCl at 162 degreesC. These reductions did not occur unless LiCl was present, illustrating the electron releasing effect of the three methoxy functions which reduce the carbonyl group's reactivity.DOI:10.1081/scc-120018935
-
作为产物:描述:iso-propyl 2-(benzo[d]thiazol-2-ylsulfonyl)acetate 在 titanium(IV) isopropylate 、 偶氮二异丁腈 、 三(三甲基硅基)硅烷 作用下, 以 乙腈 、 苯 为溶剂, 反应 13.5h, 生成 (E)-isopropyl cinnamate参考文献:名称:三取代高活化苯并[d]噻唑-2-基砜的烯烃是有机合成中的基础。摘要:在本文中,我们报告了高度亲电子的1,1-失活烯烃的形成,它们作为新型合成构件的使用以及它们向结构多样的分子支架的转化。分别被BT-磺酰基和羰基或腈取代的1,1-失活烯烃的合成由不寻常的Ti(OPr i)4组成介导的Knoevenagel型缩合反应,收率好至极好。产生的烯烃可以高度立体选择性的方式和高收率进一步转化为各种多官能化杂环和无环分子支架。总体而言,从(主要是)市售醛开始,分两到四个步骤获得所得结构。另外,在制备的结构中BT-磺酰基的存在允许进一步的化学选择性官能化/合成后转化以提供结构上多样化的最终化合物。DOI:10.1021/acs.joc.0c00571
文献信息
-
Primary Amides. A General Nitrogen Source for Catalytic Asymmetric Aminohydroxylation of Olefins作者:Zachary P. Demko、Michael Bartsch、K. Barry SharplessDOI:10.1021/ol000098m日期:2000.7.1N-Bromo,N-lithio salts of primary carboxamides have been shown to be efficient nitrogen sources for catalytic asymmetric aminohydroxylation of olefins, behaving much like the parent N-bromoacetamide in these reactions. alpha-Chloro-N-bromoacetamide is a particularly interesting nitrogen source, as it is functionalized for further reaction, including easy deprotection by treatment with thiourea.
-
Novel multi-dentate phosphines for Pd-catalyzed alkoxycarbonylation of alkynes promoted by H2O additive作者:Da Yang、Lei Liu、Dong-Liang Wang、Yong Lu、Xiao-Li Zhao、Ye LiuDOI:10.1016/j.jcat.2019.01.031日期:2019.3A series of novel multi (bi-/tri-/tetra-)-dentate phosphines with good robustness against water and oxygen were synthesized and fully characterized. It was found that the developed ionic tri-dentate phosphine (L2′) enabled Pd-catalyzed alkoxycarbonylation of alkynes most efficiently while H2O was used as an additive instead of acid. As for L2′, its unique steric configuration with two types of potential合成并充分表征了一系列新颖的多(双-/三-/四-)齿状膦,其对水和氧具有良好的耐受性。发现开发的离子型三齿膦(L2')能够最有效地Pd催化炔烃的烷氧基羰基化,同时使用H 2 O代替酸作为添加剂。至于L2',其独特的空间构型以及两种潜在的PP螯合模式(P⋯P距离分别为4.31Å和4.36Å)到Pd中心使得相应的Pd催化剂具有高活性和良好的炔烃羰基羰基化稳定性。的原位FT-IR分析也证实了钯-H活性物质的形成和稳定性大大用的存在促进L2'和H 2 O添加剂。此外,作为离子化膦,固定在[Bmim] NTf 2的RTIL中的基于L2'的PdCl 2(MeCN)2系统可以循环使用7次,而不会造成明显的活性损失或金属浸出。
-
NHC-catalyzed C–O or C–N bond formation: efficient approaches to α,β-unsaturated esters and amides作者:Bo Zhang、Peng Feng、Yuxin Cui、Ning JiaoDOI:10.1039/c2cc32862c日期:——Simple and efficient NHC-catalyzed transformations of bromoenal or α,β-dibromoenal into α,β-unsaturated esters or amides with high stereoselectivity through CâO or CâN bond formation have been demonstrated. The NHC-catalyzed processes occur under mild conditions. The ready availability of the starting materials, avoidance of external oxidants and the usefulness of the products all make the strategy attractive.
-
Tandem Oxidation Processes: The Direct Conversion of Activated Alcohols into Esters and Amides作者:Richard J. Taylor、Jonathan S. Foot、Hisashi Kanno、Gerard M. GiblinDOI:10.1055/s-2002-32951日期:——The direct conversion of primary alcohols into methyl esters and amides using manganese dioxide and sodium cyanide with methanol or the appropriate amine is reported. These transformations, which proceed via an in situ four step, double oxidation sequence, have been applied to a range of benzylic, heterocyclic, allylic and propargylic alcohols.
-
Photoinduced Oxidative Alkoxycarbonylation of Alkenes with Alkyl Formates作者:Wan-Ying Tang、Ling Chen、Ming Zheng、Le-Wu Zhan、Jing Hou、Bin-Dong LiDOI:10.1021/acs.orglett.1c01096日期:2021.5.21A photoinduced oxidative alkoxycarbonylation of alkenes initiated by intermolecular addition of alkoxycarbonyl radicals has been demonstrated. Employing alkyl formates as alkoxycarbonyl radical sources, a range of α,β-unsaturated esters were obtained with good regioselectivity and E selectivity under ambient conditions.已经证明了通过分子间加成烷氧基羰基引发的烯烃的光诱导氧化烷氧基羰基化。利用烷基甲酸酯作为烷氧羰基自由基源,在环境条件下获得了一系列具有良好区域选择性和E选择性的α,β-不饱和酯。
表征谱图
-
氢谱1HNMR
-
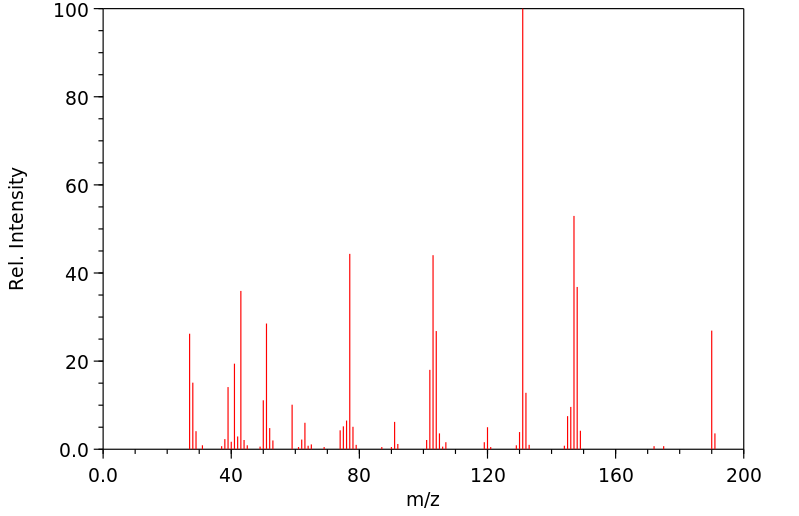
质谱MS
-
碳谱13CNMR
-
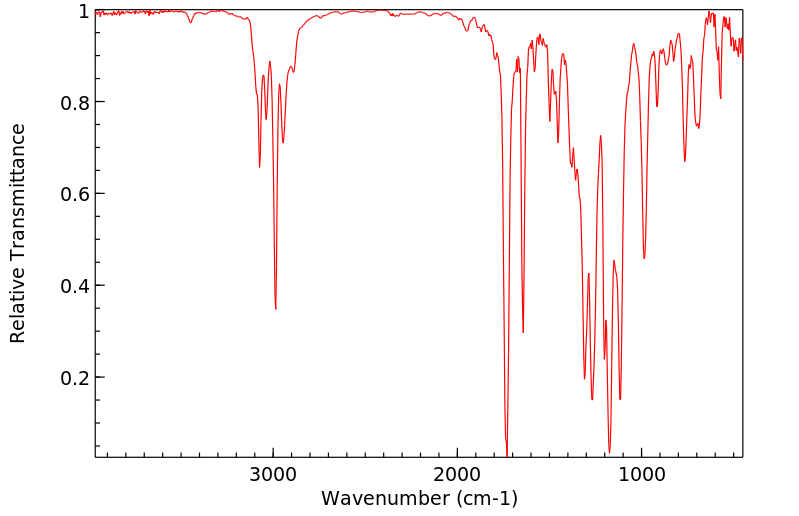
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30