sodium dibenzyldithiocarbamate | 55310-46-8
物质功能分类
中文名称
——
中文别名
——
英文名称
sodium dibenzyldithiocarbamate
英文别名
sodium dibenzylcarbamodithioate;sodium;N,N-dibenzylcarbamodithioate
CAS
55310-46-8
化学式
C15H14NS2*Na
mdl
——
分子量
295.405
InChiKey
MYCPTMLDBVIIES-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:235 °C (dec.)(lit.)
-
溶解度:在EtOH中几乎透明
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):0.52
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:36.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
危险类别码:R22
-
海关编码:2930909090
-
储存条件:请将贮藏器密封保存,并存放在阴凉、干燥处。确保工作环境有良好的通风或排气设施。
SDS
二苄基二硫代氨基甲酸钠水合物
模块 1. 化学品
产品名称: Sodium Dibenzyldithiocarbamate Hydrate
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二苄基二硫代氨基甲酸钠水合物
百分比: >98.0%(T)
CAS编码: 55310-46-8
俗名: Dibenzyldithiocarbamic Acid Sodium Salt Hydrate
分子式:
C15H14NNaS2·xH2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
二苄基二硫代氨基甲酸钠水合物
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
远离不相容的材料比如氧化剂存放。
热敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
二苄基二硫代氨基甲酸钠水合物
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
二苄基二硫代氨基甲酸钠水合物
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Sodium Dibenzyldithiocarbamate Hydrate
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二苄基二硫代氨基甲酸钠水合物
百分比: >98.0%(T)
CAS编码: 55310-46-8
俗名: Dibenzyldithiocarbamic Acid Sodium Salt Hydrate
分子式:
C15H14NNaS2·xH2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
二苄基二硫代氨基甲酸钠水合物
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
远离不相容的材料比如氧化剂存放。
热敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
二苄基二硫代氨基甲酸钠水合物
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
二苄基二硫代氨基甲酸钠水合物
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:一种制备二硫化四苄基秋兰姆的方法摘要:本发明属于橡胶助剂生产领域技术领域,涉及一种制备二硫化四苄基秋兰姆的方法。步骤如下:反应器中依次加入水、氢氧化钠水溶液、二苄胺后加入分散剂,然后滴加二硫化碳进行缩合反应,得到二苄基二硫代甲酸钠溶液;二苄基二硫代甲酸钠溶液氧化反应后过滤、干燥,得到二硫化四苄基秋兰姆。本发明在缩合反应过程中加入分散剂,反应体系形成均一的状态,反应过程在均一体系中进行,避免了现有技术中滴加二硫化碳时是二相反应,反应效果差的问题。废水中的COD含量低,易于废水处理。所得到的二硫化四苄基秋兰姆产品外观为白色粉末,纯度98%以上。公开号:CN113666855A
-
作为产物:描述:参考文献:名称:二硫代氨基甲酸盐和黄原酸盐的立体电子图谱摘要:[PdBr( i Pr 2 -bimy)(L∧X)] 类型的 12 种钯 (II) 配合物库包含 10 个二硫代氨基甲酸 (R 2 NCS 2 – ) 和两个黄原酸 (ROCS 2 – ) 配体完全表征。有了这些配合物,双齿单阴离子配体的电子和空间特性使用 HEP2 和 % V bur进行了评估方法论。此外,第一个二硫代氨基甲酸酯立体电子图的构建使得能够在原则上识别最佳配体参数,以增强其金 (III) 配合物的细胞毒活性。立体电子图的这种应用展示了它作为建立合理配体设计的结构-活性关系的有用工具的可行性。DOI:10.1021/acs.inorgchem.2c03387
文献信息
-
In vitro toxicity of several dithiocarbamates and structure-activity relationships作者:N. Segovia、G. Crovetto、P. Lardelli、M. EspigaresDOI:10.1002/jat.868日期:2002.11Dithiocarbamates (DTCs) are chemicals featuring a great chelating capacity. The toxicological study of DTCs is very important in view of their relatively simple synthesis and wide array of sanitary and industrial applications. In this study, the toxicity of some of the more recently synthesized DTCs is determined using an extremely simple bioassay, described in previous studies, based on the inhibition of growth of Escherichia coli (IGEC). The lowest-observed-effect concentration (LOEC), the median effective concentration (EC50) and no-observed-effect concentration (NOEC) of the following sodium dithiocarbamates was determined: N-benzyl-N-methyldithiocarbamate·2H2O, N-benzyl-N-isopropyldithiocarbamate·3H2O, N-benzyl-N-ethyldithiocarbamate·2H2O, N-butyl-N-methyldithiocarbamate·2H2O, N,N-dibenzyldithiocarbamate·2H2O and N-benzyl-2-phenethyldithiocarbamate·4H2O. Our results showed N,N-dibenzyl-DTC to be the least toxic of the tested substances, with an EC50 value of 1269.9 µg ml−1, whereas N-butyl-N-methyl-DTC and N-benzyl-N-methyl-DTC, with respective EC50 values of 14.9 µg ml−1 and 23.5 µg ml−1, were the most toxic. Regression analysis showed, through exponential models, that the degree of toxicity of this group of substances correlated with the molecular weight of the compound, the molecular weight of the smallest chemical radical linked to the dithiocarbamate group and the number of benzene rings present in the molecule. The consideration of these models allows us to establish that in general terms the toxicity of DTCs decreases exponentially with a greater molecular weight and the number of benzene rings. Copyright © 2002 John Wiley & Sons, Ltd.二硫代氨基甲酸盐 (DTCs) 是一类具有很强螯合能力的化学物质。鉴于其合成相对简单以及在卫生和工业应用中的广泛性,对 DTCs 的毒理学研究非常重要。在本研究中,我们使用了一种基于大肠杆菌 (IGEC) 生长抑制的极其简单的生物测定法,来确定一些较新合成的 DTCs 的毒性,该方法在先前的研究中已有描述。我们确定了以下二硫代氨基甲酸钠的最小观察效应浓度 (LOEC)、中效浓度 (EC50) 和无观察效应浓度 (NOEC):N-苄基-N-甲基二硫代氨基甲酸酯·2H2O、N-苄基-N-异丙基二硫代氨基甲酸酯·3H2O、N-苄基-N-乙基二硫代氨基甲酸酯·2H2O、N-丁基-N-甲基二硫代氨基甲酸酯·2H2O、N,N-二苄基二硫代氨基甲酸酯·2H2O 和 N-苄基-2-苯乙基二硫代氨基甲酸酯·4H2O。我们的结果显示,N,N-二苄基-DTC 是所测试物质中毒性最小的,其 EC50 值为 1269.9 µg ml^-1,而 N-丁基-N-甲基-DTC 和 N-苄基-N-甲基-DTC 的 EC50 值分别为 14.9 µg ml^-1 和 23.5 µg ml^-1,是毒性最大的。回归分析通过指数模型显示,这组物质的毒性程度与化合物的分子量、与二硫代氨基甲酸基团相连的最小化学基团的分子量以及分子中存在的苯环数量有关。这些模型的考虑使我们能够确定,从一般意义上讲,DTCs 的毒性随着分子量的增加和苯环数量的增多呈指数级下降。版权所有 © 2002 John Wiley & Sons, Ltd。
-
Reactions of Naphthyl Tellurium Trihalides with Some Dithiocarbamates and Xanthates: X-Ray Structure of “T-Shaped” Naphthyl Tellurium 1-Pyrrolidinecarbodithioate作者:Martin D. Rudd、Angela Defferding、Kevin K. KlausmeyerDOI:10.1080/10426500802202063日期:2008.9.15The reaction of naphthyl tellurium trihalides, ArTeX3 (X = Br, Cl; Ar = naphthyl) with several salts of dithiocarbamate and xanthate ligands (L) have been investigated by metathesis in tetrahydrofuran. The products (1–3) were characterized by multinuclear NMR spectroscopy and elemental analysis. Crystals of ArTeL (L = 1-pyrrolidine-carbodithioate, 1) were obtained from the reaction of ArTeCl3 and 3萘基碲三卤化物、ArTeX3(X = Br、Cl;Ar = 萘基)与几种二硫代氨基甲酸盐和黄原酸盐配体 (L) 的反应已通过四氢呋喃中的复分解进行了研究。产物(1-3)通过多核核磁共振光谱和元素分析进行了表征。ArTeL 的晶体(L = 1-吡咯烷-二硫代碳化物,1)是从 ArTeCl3 和 3 当量的 NH4[S2CNC4H8] 的反应中获得的。该结构显示了一个三坐标 T 形碲 (II) 几何结构,具有一个“短”和一个“长”Te-S 键:晶胞中两个独立的分子的 Te-S 距离为 2.444(3) Å,分别为 2.494(3) Å 和 3.271(5) Å, 3.042(5) Å,后两个距离是分子间距离。讨论了分子间相互作用和核磁共振谱,以及相关 Te(II) 配合物的结构比较。
-
Syntheses, crystal and molecular structures, and properties of some new phenylmercury(ii) dithiolate complexes作者:Nanhai Singh、Abhinav Kumar、Kieran C. Molloy、Mary F. MahonDOI:10.1039/b804635b日期:——spectrometry. The crystal structures of 1, 2 and 3 showed a linear Hg(II) core at the center of the molecules. The weak intra- and intermolecular Hg⋯S interactions provide a molecular chain framework. The reaction of PhHgO2CCH3 with Bun2dtcH gave the known dimeric complex Hg(Bun2dtc)2 while the Ni(O2CCH3)2 mediated reaction gave 1 instead of the expected heterobimetallic complex [PhHgNi(Bun2CS2)2]O2CCH3一系列新苯基汞的(II)二硫代配合物[PhHg(BU Ñ 2 DTC)](1 ;卜Ñ 2 DTC - =二-正丁基二),[PhHg(morphdtc)](2 ; morphdtc - = morpholinedithiocarbamate), [PhHg(BZ 2 DTC)](3 ;的BZ 2 DTC - =二苄基),[PhHg(methoxethxant)](4 ; methoxethxant - = 2- methoxyethylxanthate)[(PhHg)2 NED](5 ; NED 2- = 1 -硝基亚乙基-2,2-二硫代硫酸盐)和[(PhHg)2 CDC](6;CDC 2- =氰基二硫代亚氨基碳酸酯)已经制备并表征为元素分析, 紫外可见, 红外,1 H和13 C NMR谱 和 质谱。的晶体结构1,2和3呈线性汞(II在分子的中心)的核心。Hg⋯S分子内和分子间的弱相互作用提供了一个分子链框架。PhHgO
-
Neutral and Ionic Complexes of C<sub>60</sub> with Metal Dibenzyldithiocarbamates. Reversible Dimerization of C<sub>60</sub><sup>•</sup><sup>-</sup> in Ionic Multicomponent Complex [Cr<sup>I</sup>(C<sub>6</sub>H<sub>6</sub>)<sub>2</sub><sup>•</sup><sup>+</sup>]·(C<sub>60</sub><sup>•</sup><sup>-</sup>)·0.5[Pd(dbdtc)<sub>2</sub>]作者:Dmitri V. Konarev、Andrey Yu. Kovalevsky、Akihiro Otsuka、Gunzi Saito、Rimma N. LyubovskayaDOI:10.1021/ic051261i日期:2005.12.1molecular complexes of C60 with metal(II) dibenzyldithiocarbamates, M(dbdtc)2.C60.0.5(C6H5Cl), where M=Cu(II), Ni(II), Pd(II), and Pt(II) and an ionic multicomponent complex [Cr(I)(C6H6)2*+].(C60*-).0.5[Pd(dbdtc)2] (Cr(C6H6)2: bis(benzene)chromium) were obtained. According to IR, UV-visible-NIR, and EPR spectra, involve neutral components, whereas 5 comprises neutral Pd(dbdtc)2 and C60*- and Cr(I)(C6H6)2*+C60与金属(II)二苄基二硫代氨基甲酸酯金属(M(dbdtc)2.C60.0.5(C6H5Cl))的新分子配合物,其中M = Cu(II),Ni(II),Pd(II)和Pt(II)得到离子多组分络合物[Cr(I)(C6H6)2 * +]。(C60 *-)。0.5 [Pd(dbdtc)2](Cr( )2:双(苯)铬)。根据IR,UV-可见-NIR和EPR光谱涉及中性成分,而5包含中性Pd(dbdtc)2和C60 *-和Cr(I)( )2 * +自由基离子。在90 K处的晶体结构揭示了与由Pd(dbdtc)2组成的层交替出现的强烈起皱的富勒烯层。Cr(I)( )2 * +自由基阳离子排列在各层之间。富勒烯自由基阴离子在层中形成对,富勒烯C ... C接触为3.092(2)A,表明它们在90 K时处于单体状态。该接触实质上比两个碳原子的范德华半径之和短,因此,C60 *-可以
-
Experimental and Theoretical Study of Gold(III)-Catalyzed Hydration of Alkynes作者:Jesús Cordón、Gonzalo Jiménez-Osés、José M. López-de-Luzuriaga、Miguel Monge、M. Elena Olmos、David PascualDOI:10.1021/om500523r日期:2014.7.28dissociation of a labile ligand (Cl or Br) followed by coordination and activation of the alkyne, solvent-assisted attack of water, and enol tautomerization has been proposed through computational studies.
表征谱图
-
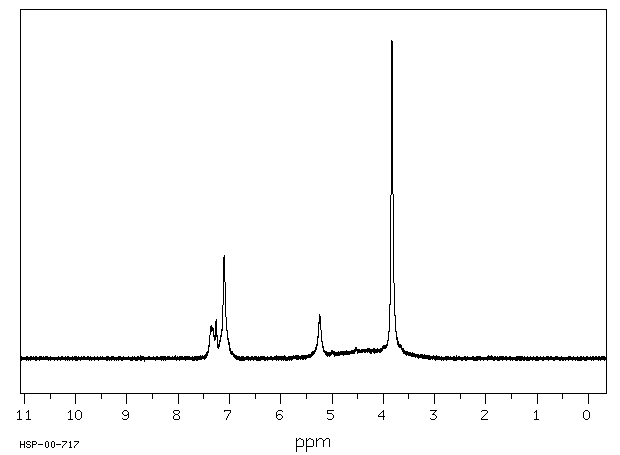
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
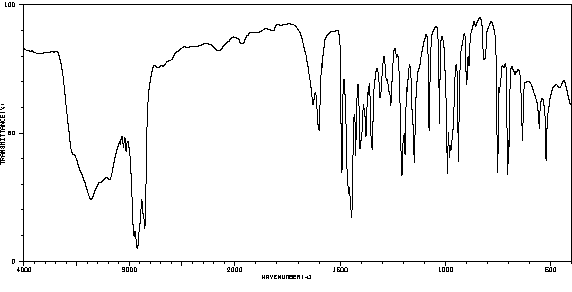
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫