α-L-sorbopyranose | 470-15-5
中文名称
——
中文别名
——
英文名称
α-L-sorbopyranose
英文别名
L-sorbose;alpha-L-Sorbopyranose;(2R,3S,4R,5S)-2-(hydroxymethyl)oxane-2,3,4,5-tetrol
CAS
470-15-5
化学式
C6H12O6
mdl
——
分子量
180.158
InChiKey
LKDRXBCSQODPBY-BGPJRJDNSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
物理描述:Solid
-
熔点:165°C
-
溶解度:360.0 mg/mL at 17 °C
计算性质
-
辛醇/水分配系数(LogP):-2.8
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:110
-
氢给体数:5
-
氢受体数:6
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 可得然胶 β-D-glucose 492-61-5 C6H12O6 180.158 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl α-L-sorbopyranoside 3765-95-5 C7H14O6 194.185 —— methyl 4,6-O-isopropylidene-α-L-sorbofuranoside 22595-85-3 C10H18O6 234.249 —— L-xylo-hexulosonic acid methyl ester 72933-30-3 C7H12O7 208.168 2,3-O-(1-甲基乙亚基)-alpha-L-呋喃山梨糖 2,3-O-isopropylidene-α-L-sorbofuranose 17682-71-2 C9H16O6 220.222 —— 11,2:4,6-di-O-isopropylidene-α-L-sorbofuranose 18604-19-8 C12H20O6 260.287 双丙酮-L-山梨糖 2,3:4,6-bis-O-isopropylidene-L-sorbose 17682-70-1 C12H20O6 260.287
反应信息
-
作为反应物:描述:参考文献:名称:Khouvine; Arragon, Bulletin de la Societe Chimique de France, 1938, vol. <5>5, p. 1410摘要:DOI:
-
作为产物:描述:参考文献:名称:更严格的限制增加了钛沸石中d-葡萄糖向l-山梨糖异构化的选择性。摘要:d-葡萄糖的水相异构化为d-果糖和l-山梨糖被硅质骨架中的Lewis酸性Ti位点平行催化。当Ti位点限制在介孔空隙(Ti‐MCM‐41,TiO 2 ‐ SiO 2)中时,无法检测到葡萄糖异构化速率(每Ti,373 K))并将Ti位置限制在Ti-Beta的较小的12元环(12-MR)微孔中,并增加到可检测的值。由于Ti位置被限制在10-MR孔(Ti-MFI,Ti-CON)内,异构化速率降低至较低值(≈20x),并且微孔尺寸进一步减小,这可能是由于孔内反应物扩散限制所致,并达到了无法检测的值在Ti-CHA的8-MR孔中,因为尺寸排阻阻止了葡萄糖进入活性位点。值得注意的是,相对于d,对l-山梨糖的选择性随着Ti部位周围空间的限制变得越来越严格,果糖系统地增加,而在Ti-MFI上果糖则大于10。这些发现证明了在Ti活性位点附近的限制对平行立体选择性糖异构化途径之间选择性的显着影响。DOI:10.1002/anie.202005207
文献信息
-
Catalytic asymmetric epoxidation申请人:——公开号:US06348608B1公开(公告)日:2002-02-19A compound and method for producing an enantiomerically enriched epoxide from an olefin using a chiral ketone and an oxidizing agent is disclosed.一种化合物及其生产方法被披露,该方法使用手性酮和氧化剂从烯烃生产具有对映体富集的环氧乙烷。
-
An Efficient Catalytic Asymmetric Epoxidation Method作者:Zhi-Xian Wang、Yong Tu、Michael Frohn、Jian-Rong Zhang、Yian ShiDOI:10.1021/ja972272g日期:1997.11.1This article describes a highly effective catalytic asymmetric epoxidation method for olefins using potassium peroxomonosulfate (Oxone, Dupont) as oxidant and a fructose-derived ketone (1) as catalyst. High enantioselectivies have been obtained for trans-disubstituted and trisubstituted olefins which can bear functional groups such as tributylsilyl ether, acetal, chloride, and ester. The enantiomeric
-
Synthesis of l-fructose作者:Chyi-Cheng Chen、Roy L. WhistlerDOI:10.1016/0008-6215(88)84148-x日期:1988.5-sorbofuranoside, which was tosylated to give methyl 4,6- O -isopropylidene-1,3-di- O - p -tolylsulfonyl-α- l -sorbofuranoside. Removal of the isopropylidene protecting group and formation of a 3,4-anhydro-ring afforded methyl 3,4-anhydro-1- O - p -tolyl-sulfonyl-α- l -tagatofuranoside, which was converted into l -fructose ( 7 ) by acid hydrolysis. In an alternative, facile synthesis, 1 was converted, in
-
Efficient One-Pot Synthesis of 5-Chloromethylfurfural (CMF) from Carbohydrates in Mild Biphasic Systems作者:Wenhua Gao、Yiqun Li、Zhouyang Xiang、Kefu Chen、Rendang Yang、Dimitris ArgyropoulosDOI:10.3390/molecules18077675日期:——5-Halomethylfurfurals can be considered as platform chemicals of high reactivity making them useful for the preparation of a variety of important compounds. In this study, a one-pot route for the conversion of carbohydrates into 5-chloromethylfurfural (CMF) in a simple and efficient (HCl-H3PO4/CHCl3) biphasic system has been investigated. Monosaccharides such as D-fructose, D-glucose and sorbose, disaccharides such as sucrose and cellobiose and polysaccharides such as cellulose were successfully converted into CMF in satisfactory yields under mild conditions. Our data shows that when using D-fructose the optimum yield of CMF was about 47%. This understanding allowed us to extent our work to biomaterials, such as wood powder and wood pulps with yields of CMF obtained being comparable to those seen with some of the enumerated mono and disaccharides. Overall, the proposed (HCl-H3PO4/CHCl3) optimized biphasic system provides a simple, mild, and cost-effective means to prepare CMF from renewable resources.5 -氯甲基糠醛可被视为具有高反应活性的平台化学品,可用于制备多种重要化合物。本研究探讨了在简单高效的(HCl-H3PO4/CHCl3)双相体系中将碳水化合物转化为 5-氯甲基糠醛(CMF)的一步法路线。在温和的条件下,D-果糖、D-葡萄糖和山梨糖等单糖,蔗糖和纤维二糖等二糖,以及纤维素等多糖被成功转化为 CMF,产量令人满意。我们的数据显示,使用 D-果糖时,CMF 的最佳产量约为 47%。有了这一认识,我们就可以将工作范围扩大到生物材料,如木粉和木浆,所获得的 CMF 产率与使用某些列举的单糖和二糖所获得的产率相当。总之,所提出的(HCl-H3PO4/ )优化双相体系为利用可再生资源制备 CMF 提供了一种简单、温和且经济有效的方法。
-
CONVERSION OF GLUCOSE TO SORBOSE申请人:CALIFORNIA INSTITUTE OF TECHNOLOGY公开号:US20140309415A1公开(公告)日:2014-10-16The present invention is directed to methods for preparing sorbose from glucose, said method comprising: (a) contacting the glucose with a silica-containing structure comprising a zeolite having a topology of a 12 membered-ring or larger, an ordered mesoporous silica material, or an amorphous silica, said structure containing Lewis acidic Ti 4+ or Zr 4+ or both Ti 4+ and Zr 4+ framework centers, said contacting conducted under reaction conditions sufficient to isomerize the glucose to sorbose. The sorbose may be (b) separated or isolated; or (c) converted to ascorbic acid.
表征谱图
-
氢谱1HNMR
-
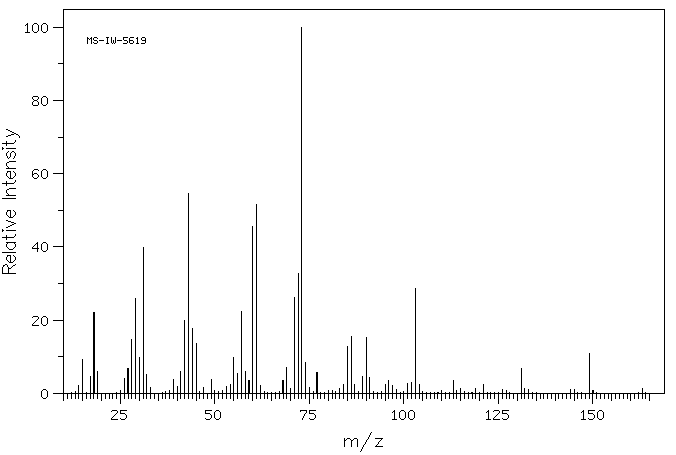
质谱MS
-
碳谱13CNMR
-
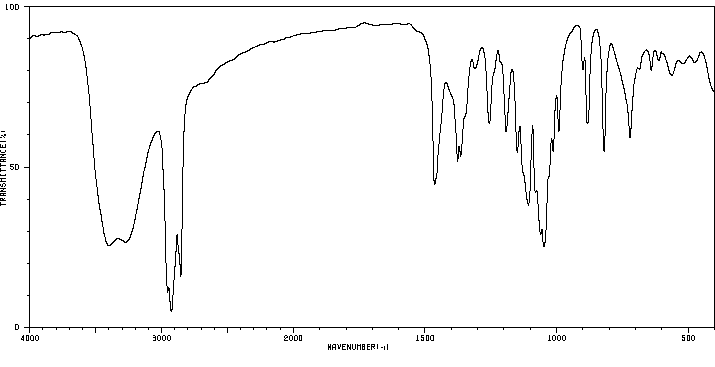
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷