liothyronine sodium | 55-06-1
物质功能分类
中文名称
——
中文别名
——
英文名称
liothyronine sodium
英文别名
sodium;4-[4-[(2S)-2-amino-2-carboxyethyl]-2,6-diiodophenoxy]-2-iodophenolate
CAS
55-06-1
化学式
C15H11I3NO4*Na
mdl
——
分子量
672.96
InChiKey
SBXXSUDPJJJJLC-YDALLXLXSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:205 °C (dec.)(lit.)
-
比旋光度:20 º (c=2,alcohol/1.2N HCl)
-
溶解度:MNH4OH甲醇溶液:125 g/5mL,澄清,黄棕色
-
碰撞截面:205.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):-0.38
-
重原子数:24
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:95.6
-
氢给体数:2
-
氢受体数:5
安全信息
-
危险品标志:Xn
-
安全说明:S22,S24/25
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2922509090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:本品应密封存于0℃以下干燥、避光的环境中保存。
SDS
| Name: | 3 3 5- Triiodo-L-Thyronine Sodium Salt 95% Material Safety Data Sheet |
| Synonym: | Liothyronine Sodium |
| CAS: | 55-06-1 |
Synonym:Liothyronine Sodium
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 55-06-1 | 3,3',5- Triiodo-L-Thyronine, Sodium Sa | 95% | 200-223-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Hygroscopic (absorbs moisture from the air).The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Hormones and antibiotics room. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 55-06-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 205 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H11I3NO4Na
Molecular Weight: 672.96
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants, exposure to moist air or water.
Incompatibilities with Other Materials:
Moisture, oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen, hydrogen iodide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 55-06-1: YP2836500 LD50/LC50:
Not available.
Carcinogenicity:
3,3',5- Triiodo-L-Thyronine, Sodium Salt - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 55-06-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 55-06-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 55-06-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
碘塞罗宁钠简介
碘塞罗宁钠为白色或类白色的粉末状物,是一种人工合成的三碘甲状腺原氨酸钠。其作用与甲状腺素相似,可作为内源性甲状腺激素的替代物。
药理作用本品是人工合成制剂,药理作用类似于甲状腺素,但药效是内源性甲状腺素的3~5倍。口服后吸收良好,蛋白结合率较高,在体内约0.3%以游离形式存在。血中半衰期为1~2日,若甲状腺功能较低时则半衰期会延长;反之,则不到一日。
药动学T3钠盐胃肠道吸收完全,与T4相比,T3与血浆蛋白的结合程度较低,约0.3%以游离形式存在。在甲状腺功能正常情况下,T3在血中的半衰期约为1~2天;在甲状腺功能减退时略延长,在甲状腺功能亢进时约为0.6天。
适应证用于需要迅速见效的甲状腺功能减退及黏液性水肿昏迷患者,也可用于甲状腺功能亢进症的诊断。
剂量与用法- 口服:
- 静脉注射:黏液性水肿昏迷患者初始剂量40~120μg,以后每6小时5~15μg。直至患者清醒时改为口服。
- 甲状腺激素如用量适当无任何不良反应。使用过量则引起心动过速、心悸、心绞痛、心律失常、头痛、神经质、兴奋、不安、失眠、骨路肌痉挛、肌无力、震颤、出汗、潮红、怕热、发热、腹泻、呕吐、体重减轻等类似甲状腺功能亢进的症状。T3过量时,不良反应的发生比T4或甲状腺粉更快,减量或停药可使所有症状消失。T4过量所致者,症状消失较缓慢。
- 长期大剂量可引起心悸,手震颤,出汗,体重减轻,神经兴奋性升高和失眠。老年和心脏病人可引发心绞痛、心肌梗塞、心源性虚脱,此时应停用本品。
- 不良反应:心血管系统有心悸、心动过速、心绞痛,偶见心律失常与心衰,故冠心病、高血压患者慎用本品;中枢神经系统有头痛、兴奋、失眠、神经质,偶见昏迷;消化系统有恶心、呕吐及腹泻等;其他有肌无力、新陈代谢加快(皮肤潮红、发热、出汗、怕热、体重减轻),偶见死亡。
- 本品可会使抗凝剂及三环类抗抑郁药的药效增强。
- 糖尿病患者服用本品时,应调整好降糖药的剂量,适量增加。
- 普萘洛尔、胺碘酮可阻止甲状腺素转化成本品,进而导致T3浓度降低与无活性的反T3浓度增高。
- 若发生急性药物过量时,应立即采取措施,减少药物在胃肠道中的滞留,进而减少机体对药物的吸收,同时还应进行对症治疗和支持治疗。
- 本品不作为一般替代治疗的首选药。
- 片剂:20μg
- 粉针剂:20μg
[1] 刘福强等 主编. 医师案头用药参考. 北京:中国中医药出版社, 2012.
文献信息
-
ANTHELMINTIC COMPOUNDS AND COMPOSITIONS AND METHOD OF USING THEREOF申请人:Meng Charles Q.公开号:US20140142114A1公开(公告)日:2014-05-22The present invention relates to novel anthelmintic compounds of formula (I) below: wherein Y and Z are independently a bicyclic carbocyclic or a bicyclic heterocyclic group, or one of Y or Z is a bicyclic carbocyclic or a bicyclic heterocyclic group and the other of Y or Z is alkyl, alkenyl, alkynyl, cycloalkyl, phenyl, heterocyclyl or heteroaryl, and variables X 1 , X 2 , X 3 , X 4 , X 5 , X 6 , X 7 and X 8 are as defined herein. The invention also provides for veterinary compositions comprising the anthelmintic compounds of the invention, and their uses for the treatment and prevention of parasitic infections in animals.本发明涉及以下式(I)的新型驱虫化合物: 其中 Y和Z分别是双环碳环或双环杂环基团,或者Y或Z中的一个是双环碳环或双环杂环基团,另一个是烷基,烯基,炔基,环烷基,苯基,杂环基或杂芳基,以及变量X 1 ,X 2 ,X 3 ,X 4 ,X 5 ,X 6 ,X 7 和X 8 如本文所定义。本发明还提供了包含本发明的驱虫化合物的兽药组合物,以及它们用于治疗和预防动物寄生虫感染的用途。
-
[EN] ANTHELMINTIC DEPSIPEPTIDE COMPOUNDS<br/>[FR] COMPOSÉS DEPSIPEPTIDIQUES ANTHELMINTHIQUES申请人:MERIAL INC公开号:WO2018093920A1公开(公告)日:2018-05-24The present invention provides cyclic depsipeptide compounds of formula (I) wherein the stereochemical configuration of at least one carbon atom bearing the groups Cy1, Cy2, R1, R2, R3, R4, Ra and Rb is inverted compared with the naturally occurring cyclic depsipeptide PF1022A. The invention also provides compositions comprising the compounds that are effective against parasites that harm animals. The compounds and compositions may be used for combating parasites in or on mammals and birds. The invention also provides for an improved method for eradicating, controlling and preventing parasite infestation in birds and mammals.
-
Benzoxazepinones and their use as squalene synthase inhibitors申请人:——公开号:US20030078251A1公开(公告)日:2003-04-24There is disclosed a compound represented by the formula [I]: 1 wherein R 1 is optionally substituted 1-carboxyethyl group, optionally substituted alkyl-sulfonyl group, optionally substituted (carboxy-cycloalkyl)-alkyl group, —X 1 —X 2 —Ar—X 3 —X 4 —COOH (wherein X 1 and X 4 are a bond or alkylene group, X 2 and X 3 are a bond, —O—, —S—, Ar is divalent aromatic group etc.), R 2 is alkyl group optionally substituted with alkanoyloxy group and/or hydroxy group, R 3 is alkyl group, and W is halogen atom, etc., or a salt thereof. The compound has the cholesterol lowering activity and the triglyceride lowering activity and is useful for preventing and/or treating hyperlipidemia.
-
BENZAZEPINE DERIVATIVE, PROCESS FOR PRODUCING THE SAME, AND USE申请人:Takeda Chemical Industries, Ltd.公开号:EP1422228A1公开(公告)日:2004-05-26The present invention provides a novel benzazepine derivative represented by formula : wherein, R1 is a 5- or 6-membered aromatic ring, R2 is lower alkyl group, etc., Y is an optionally substituted imino group, ring A and ring B are independently an optionally substituted aromatic ring, W is formula -W1-X2-W2- (W1 and W2 are independently S(O)m1 (m1 is 0, 1 or 2), etc., and X2 is an optionally substituted alkylene groupetc. ), a preparation method and use thereof.
-
[EN] ANTI PARASITIC DIHYDROAZOLE COMPOUNDS AND COMPOSITIONS COMPRISING SAME<br/>[FR] DIHYDROAZOLES ANTIPARASITAIRES ET COMPOSITIONS LES INCLUANT申请人:MERIAL LTD公开号:WO2011075591A1公开(公告)日:2011-06-23The present invention relates to novel dihydroazole of formula (I) and salts thereof: Wherein R1, A1, A2, G, X and Y are as defined in the description, compositions thereof, processes for their preparation and their uses to prevent or treat parasitic infections or infestations in animals and as pesticides.
表征谱图
-
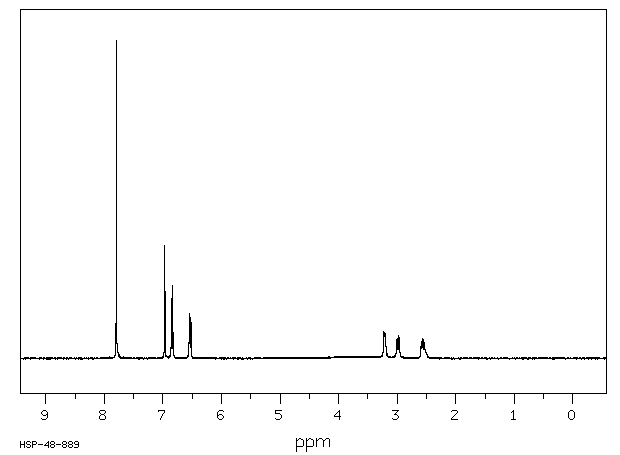
氢谱1HNMR
-
质谱MS
-
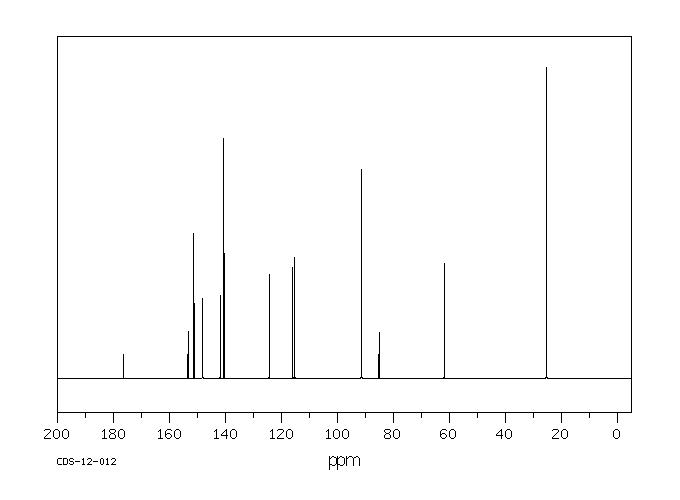
碳谱13CNMR
-
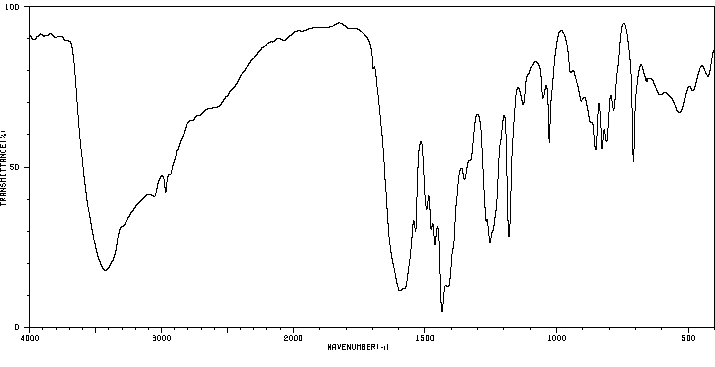
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸