5-乙酰基-2,3-异亚丙基腺苷酸 | 15888-38-7
分子结构分类
中文名称
5-乙酰基-2,3-异亚丙基腺苷酸
中文别名
5'-乙酰基-2',3'-异亚丙基腺苷;5'-O-乙酰基-2'3'-O-异亚丙基腺苷
英文名称
5'-acetyl-2',3'-isopropylideneadenosine
英文别名
acetic acid 6-(6-amino-purin-9-yl)-2,2-dimethyl-tetrahydro-furo[3,4-d][1,3]dioxol-4-ylmethyl ester;5’-O-acetyl-2’,3’-O-isopropylideneadenosine;5'-O-acetyl-2',3'-O,O-isopropylideneadenosine;2',3'-O-isopropylidene-5'-O-acetyladenosine;5'-O-acetyl-2',3'-O-isopropylideneadenosine;5'-acetyl-2',3'-O-isopropylidene adenosine;[(3aR,4R,6R,6aR)-4-(6-aminopurin-9-yl)-2,2-dimethyl-3a,4,6,6a-tetrahydrofuro[3,4-d][1,3]dioxol-6-yl]methyl acetate
CAS
15888-38-7
化学式
C15H19N5O5
mdl
——
分子量
349.346
InChiKey
AGPPMENETHBDES-IDTAVKCVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:170-172°C
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:25
-
可旋转键数:4
-
环数:4.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:124
-
氢给体数:1
-
氢受体数:9
安全信息
-
海关编码:2934999090
-
危险性防范说明:P262
-
危险性描述:H302
-
储存条件:-20°C
SDS
Version 1.2
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 5'-ACETYL-2',3'-ISOPROPYLIDENEADENOSINE,
98%
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
2',3'-O-ISOPROPYLIDENEADENOSINE 15888-38-7 240-026-1 None
5'-MONOACETATE
Formula C15H19N5O5
Molecular Weight 349,3500 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact, immediately flush eyes with copious amounts
of water for at least 15 minutes.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
ALDRICH www.molbase.com
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid inhalation. Avoid contact
with eyes, skin, and clothing. Avoid prolonged or repeated
exposure.
STORAGE
Conditions of Storage: Keep tightly closed. Store in a cool dry
place.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling. Wash contaminated clothing before
reuse.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Respiratory protection is not required. Where
protection is desired, use multi-purpose combination (US) or type
ABEK (EN 14387) respirator cartridges.
Hand Protection: Compatible chemical-resistant gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
pH N/A
BP/BP Range N/A
MP/MP Range 170,000. - 172,000 °
C.
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
ALDRICH www.molbase.com
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides.
11 - Toxicological Information
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Eye Contact: May cause eye irritation.
Multiple Routes: May be harmful by inhalation, ingestion, or
skin absorption.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
ALDRICH www.molbase.com
15 - Regulatory Information
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 5'-ACETYL-2',3'-ISOPROPYLIDENEADENOSINE,
98%
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
2',3'-O-ISOPROPYLIDENEADENOSINE 15888-38-7 240-026-1 None
5'-MONOACETATE
Formula C15H19N5O5
Molecular Weight 349,3500 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact, immediately flush eyes with copious amounts
of water for at least 15 minutes.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
ALDRICH www.molbase.com
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid inhalation. Avoid contact
with eyes, skin, and clothing. Avoid prolonged or repeated
exposure.
STORAGE
Conditions of Storage: Keep tightly closed. Store in a cool dry
place.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling. Wash contaminated clothing before
reuse.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Respiratory protection is not required. Where
protection is desired, use multi-purpose combination (US) or type
ABEK (EN 14387) respirator cartridges.
Hand Protection: Compatible chemical-resistant gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
pH N/A
BP/BP Range N/A
MP/MP Range 170,000. - 172,000 °
C.
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
ALDRICH www.molbase.com
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Nitrogen oxides.
11 - Toxicological Information
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Eye Contact: May cause eye irritation.
Multiple Routes: May be harmful by inhalation, ingestion, or
skin absorption.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
ALDRICH www.molbase.com
15 - Regulatory Information
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2',3'-异丙叉腺苷 2',3'-isopropylidene adenosine 362-75-4 C13H17N5O4 307.309 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2',3'-异丙叉腺苷 2',3'-isopropylidene adenosine 362-75-4 C13H17N5O4 307.309 —— 5'-O-acetyladenosine 2140-25-2 C12H15N5O5 309.282 —— O5'-(4,4'-dimethoxy-trityl)-O2',O3'-isopropylidene-adenosine 68074-34-0 C34H35N5O6 609.682 —— N6,5'-O-di-p-toluyl-2',3'-O-isopropylideneadenosine 93135-72-9 C29H29N5O6 543.579 —— 2',3'-O-isopropylidene-N6,N6,5'-O-tri-p-toluyladenosine 51008-69-6 C37H35N5O7 661.714
反应信息
-
作为反应物:描述:参考文献:名称:Simple method for fast deprotection of nucleosides by triethylamine-catalyzed methanolysis of acetates in aqueous medium摘要:A straightforward methodology for deacetylation of protected ribonucleosides was developed based on triethylamine-catalyzed solvolysis in aqueous methanol. Reactions are completed in a few minutes under microwave irradiation and the free nucleosides are obtained in high yield after simple evaporation of volatiles. Other important features include the involvement of readily available reagents and the compatibility with diverse functional groups, which make this process very attractive for broad application.DOI:10.1590/s0103-50532010000500013
-
作为产物:描述:在 Schwartz's reagent 作用下, 以 四氢呋喃 为溶剂, 以53%的产率得到5-乙酰基-2,3-异亚丙基腺苷酸参考文献:名称:Schwartz试剂促进受保护核苷和核苷酸的化学选择性N-脱乙酰化。摘要:涉及N-乙酰基的保护和脱保护策略被广泛用于核苷和核苷酸化学中。在本文中,我们提出了一种温和的选择性N-脱乙酰化方法,该方法可通过Schwartz试剂与嘌呤和嘧啶核苷一起使用,与核苷化学中使用的大多数常见保护基团兼容。DOI:10.1080/15257770.2015.1075552
文献信息
-
Solventless Protocol for Efficient Bis-<i>N</i>-Boc Protection of Adenosine, Cytidine, and Guanosine Derivatives作者:Siddharth A. Sikchi、Philip G. HultinDOI:10.1021/jo060430t日期:2006.8.1A solvent-free reaction employing a simple low-energy ball mill apparatus converts the amino groups of adenosine, 2-deoxyadenosine, cytidine, 2-deoxycytidine, guanosine, and 2-deoxyguanosine as well as some of their ribosyl O-protected derivatives to the corresponding bis-N-Boc carbamates. In the case of guanosine compounds, the carbonyl group of the base moiety was also blocked as its O-Boc enol carbonate
-
Automated Nanomole-Scale Reaction Screening toward Benzoate Bioisosteres: A Photocatalyzed Approach to Highly Elaborated Bicyclo[1.1.1]Pentanes作者:James J. Mousseau、Matthew A. Perry、Mark W. Bundesmann、Gary M. Chinigo、Chulho Choi、Gary Gallego、Robert W. Hicklin、Susan Hoy、David C. Limburg、Neal W. Sach、Yuan ZhangDOI:10.1021/acscatal.1c05076日期:2022.1.7Through the application of high-throughput nanoscale optimization, a mild, photocatalyzed, Minisci-like protocol was developed to access highly functionalized 1,3-disubstituted bicyclopentanes. The benzoate-isosteric compounds were prepared using a readily available organic photocatalyst, mitigating the need for precious metals. The strategy described furnished products in synthetically useful yields
-
Protection of the Amino Group of Adenosine and Guanosine Derivatives by Elaboration into a 2,5-Dimethylpyrrole Moiety作者:Ireneusz Nowak、Morris J. RobinsDOI:10.1021/ol035264f日期:2003.9.1[reaction: see text] Protection of the amino group of adenine and guanine nucleosides was effected by heating the substrates in 2,5-hexanedione. The resulting 2,5-dimethylpyrrole adducts were stable toward bases but were hydrolyzed with TFA/H(2)O to regenerate the amino function.
-
Clay‐Mediated Selective Hydrolysis of 5′‐<i>O</i>‐Acetyl‐2′,3′‐isopropylidene/Cyclohexylidene Nucleosides作者:Mukund K. Gurjar、Dhananjoy Mondal、S. V. Ravindranadh、Mukund S. ChorghadeDOI:10.1080/00397910600639968日期:2006.8Abstract A generalized procedure for the selective hydrolysis of 5′‐O‐acetyl‐2′,3′‐isopropylidene/cyclohexylidene nucleosides with a solid catalyst, Montmorillonite K‐10 in refluxing methanol, to furnish 5′‐O‐acetyl‐nucleosides is described.
-
ANTI-WRINKLE COSMETIC COMPOSITION
表征谱图
-
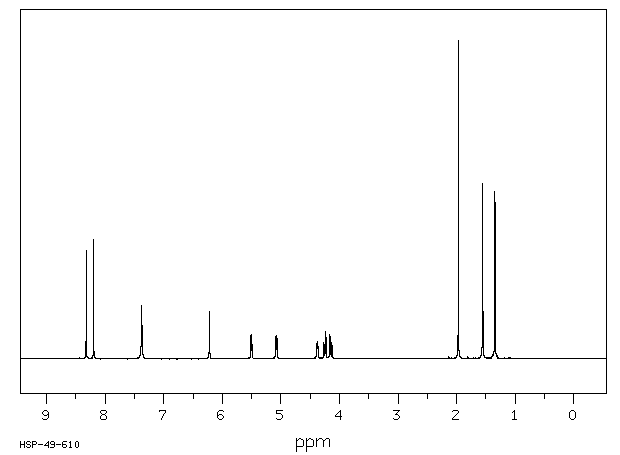
氢谱1HNMR
-
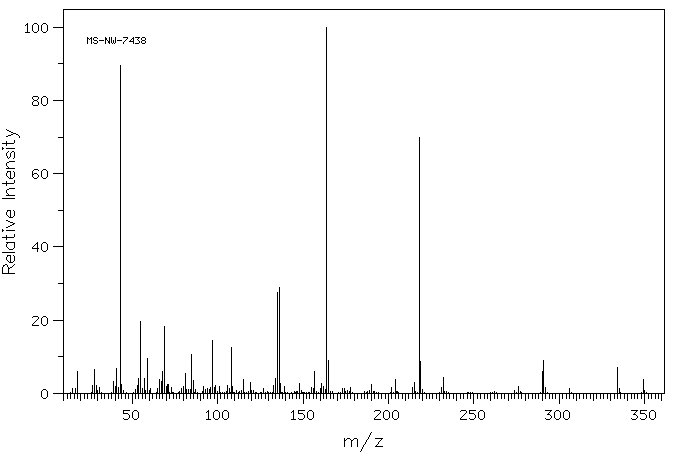
质谱MS
-
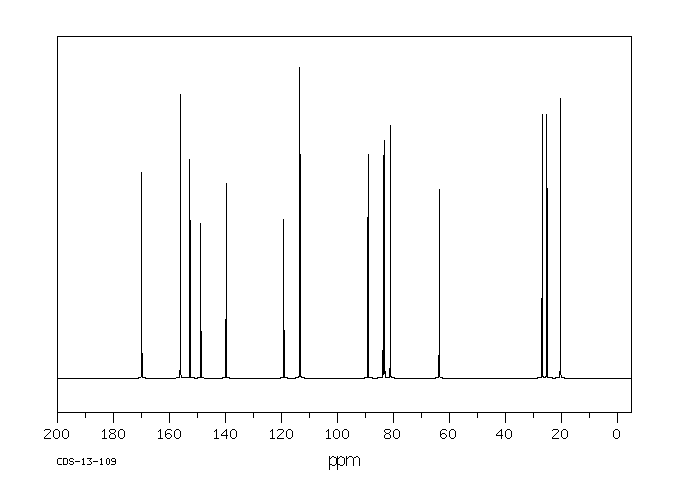
碳谱13CNMR
-
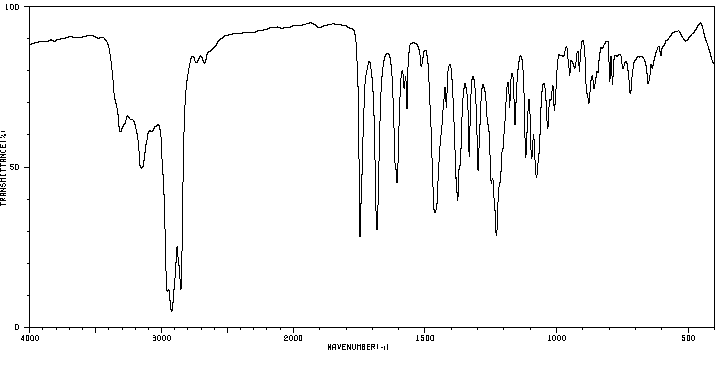
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄质核苷
黄嘌呤核苷
鸟苷5'-硫代二磷酸酯
鸟苷,N,N-二甲基-6-O-[2-(4-硝基苯基)乙基]-
鸟苷 2',3',5'-三苯甲酸酯
鸟苷
马兜铃内酰胺A
顺式-玉米素-D-核糖甙
阿糖腺苷2',3',5'-三乙酸酯
阿糖腺苷 2',3'-二乙酸酯
阿糖腺苷
阿糖腺苷
阿糖肌苷
阿洛酮糖腺苷
阿斯卡霉素
虫草素
苯酰胺,N-[9-[2-脱氧-2-氟-3,5-二-O-(四氢-2H-吡喃-2-基)-β-D-呋喃阿拉伯糖基]-9H-嘌呤-6-基]-
苯酚,4-[4-(2-氨基乙基)-2-碘苯氧基]-,盐酸(1:1)
苏云金素
腺苷酸-5-羧酸
腺苷胺类
腺苷地尔
腺苷6-N-氨基磷酸酯
腺苷5-O-硫一磷酸二锂盐
腺苷5'-O-(1-硫代二磷酸酯)
腺苷-N(6)-二乙硫基醚-N'-吡啶氧杂亚胺5'-磷酸酯
腺苷-5'-羧酸2-氯乙酯
腺苷-5'-单烟酸酯
腺苷-5'-13C
腺苷-3(+2')-单磷酸单水合物
腺苷-2,8-T2
腺苷-2'-13C
腺苷-15NN1-氧化物
腺苷,8-(丁基氨基)-N-环戊基-
腺苷,2-溴-
腺苷,2-氯-N-环丙基-
腺苷,2-[(4-甲代戊基)氧代]-
腺苷,2-(苯基甲氧基)-
腺苷,2-(环己三烯并氧基)-
腺苷,2-(2-乙基丁氧基)-
腺苷,2',3'-O-(1-甲基亚乙基)-,5'-氨基磺酸酯
腺苷
肌苷肟
肌苷-8-14C
肌苷
美他卡韦
硒-(4-硝基苯甲酰基)-6-硒肌苷
甲基4,5-二溴-3-氟噻吩-2-羧酸酯
瑞加德松杂质3
瑞加德松杂质1