肼,[(4-苯氧基苯基)甲基]- | 770-62-7
中文名称
肼,[(4-苯氧基苯基)甲基]-
中文别名
——
英文名称
trans-8a-Methyl-octahydro-1(2H)-naphthalinon
英文别名
methyl-1 bicyclo(4.4.0)decanone-2 trans;decahydro-8a-methylnaphthalenone;trans-9-methyl-1-decalone;9-methyl-1-decalone;(+/-)-8a-methyl-trans-octahydro-naphthalen-1-one;(+/-)-8a-Methyl-trans-octahydro-naphthalin-1-on;(4aR,8aS)-8a-methyl-2,3,4,4a,5,6,7,8-octahydronaphthalen-1-one
CAS
770-62-7;937-77-9;6102-35-8;6102-38-1;59981-99-6;59982-00-2;80845-00-7;91280-39-6;105561-34-0
化学式
C11H18O
mdl
——
分子量
166.263
InChiKey
DEOBYOFNFVUIGX-KOLCDFICSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
上下游信息
反应信息
-
作为反应物:描述:肼,[(4-苯氧基苯基)甲基]- 在 copper(l) iodide 、 溴 、 氯化铵 、 calcium carbonate 作用下, 以 乙醚 、 N,N-二甲基乙酰胺 、 水 、 溶剂黄146 、 N,N-二甲基甲酰胺 为溶剂, 反应 25.0h, 生成 cyano-4β dimethyl-1β,4α bicyclo(4.4.0)decanone-2 trans参考文献:名称:Stéréochimie—LIV摘要:DOI:10.1016/s0040-4020(01)97659-8
-
作为产物:描述:(8aS)-1,1-(1,2-ethylenedioxy)-1,2,3,4,6,7,8,8a-octahydro-8a-methyl-6-oxonaphthalene 在 盐酸 、 氢氧化钾 、 氨 、 lithium 、 肼 作用下, 以 四氢呋喃 、 叔丁醇 为溶剂, 反应 15.0h, 生成 肼,[(4-苯氧基苯基)甲基]-参考文献:名称:Indole diterpene synthetic studies. 2. First-generation total synthesis of (-)-paspaline摘要:DOI:10.1021/jo00275a035
文献信息
-
Chemoselective Oxidation of Equatorial Alcohols with N-Ligated λ<sup>3</sup>-Iodanes作者:Myriam Mikhael、Sophia A. Adler、Sarah E. WengryniukDOI:10.1021/acs.orglett.9b02018日期:2019.8.2of nitrogen-ligated (bis)cationic λ3-iodanes (N-HVIs) for alcohol oxidation and their unprecedented levels of selectivity for the oxidation of equatorial over axial alcohols. The conditions are mild, and the simple pyridine-ligated reagent (Py-HVI) is readily synthesized from commercial PhI(OAc)2 and can be either isolated or generated in situ. Conformational selectivity is demonstrated in both flexible
-
α-alkylation and α-alkylidenation of carbonyl compounds by o-silylated enolate phenylthioalkylation作者:Ian PatersonDOI:10.1016/s0040-4020(01)86667-9日期:1988.1For many reactions next to a carbonyl group, the use of O-silylated enolate chemistry offers improvements in yield and selectivity over the corresponding reactions of Group I metal enolates. In the case of α-alkylation of carbonyl compounds, Lewis-acid (TiCL4 or ZnBr2) promoted phenylthioalkylation of O-silylated enolates 3 by α-chlorosulphides 4 (R3=H, Me, Prn, Pri, But, and Me3Si), followed by reductive
-
Réactivité nucléophile des cyclanones: influence de la stéréochimie <i>cis</i>/<i>trans</i> en série bicyclique作者:C. Agami、T. Rizk、R. Durand、P. GenesteDOI:10.1139/v82-336日期:1982.9.15
The relative reactivities of a number of cis/trans pairs of bicyclic ketones in the 1- and 2-decalone series (2-decalone; 10-methyl-2-decalone; 9-methyl-1-decalone; and 3,10-dimethyl-2-decalone) have been studied for the nucleophilic addition of hydroxylamine in acidic medium. The results obtained as well as the rate constants for neutral or acid catalysed addition always reveal a slight preference for the trans-isomer (× 2.4). This result agrees with the proposal that preferential equatorial attack of a nucleophile is caused by conformational factors related to a steroid [Formula: see text] non-steroid conformation equilibrium in the cis-isomers.
[Journal Translation]
-
Reaktionen mit Mikroorganismen. 14. Mitteilung [1] Reduktion von stereoisomeren Dekalonen-(1) und einigen verwandten bicyclischen und tricyclischen Ketonen durchCurvularia falcata作者:W. Acklin、V. Prelog、F. Schenker、B. Serdarevi?、P. WalterDOI:10.1002/hlca.19650480733日期:——Racemic, stereoisomeric decal-1-ones and several related bicyclic and tricyclic ketones have been reduced microbiologically with high stereospecificity by Curvularia falcata (TEHON) BOEDIJN. The constitution, and the relative and absolute configuration, of the products have been determined.
-
Synthesis and Biological Evaluation of Subglutinol Analogs for Immunomodulatory Agents作者:Hyeri Park、Laura S. Christian、Mi Jung Kim、Qi-Jing Li、Jiyong HongDOI:10.1021/acs.jmedchem.9b01579日期:2020.1.9requirements of subglutinols for immunomodulatory activity and to provide guiding principles on future therapeutic development, we prepared and evaluated several subglutinol analogs for their immunomodulatory activities. Our efforts identified a subglutinol analog with reduced structural complexity as a potential lead compound for future autoimmune drug development. Our study will provide an important自身免疫性疾病是与高发病率和高死亡率相关的慢性炎症性疾病。在过去的几十年中,自身免疫性疾病的治疗选择有所增加,但是由于高毒性和缺乏选择性,它们的临床疗效普遍受到限制。因此,必须努力确定通过新机制有效规避现有副作用的新的免疫调节剂。为了定义亚谷蛋白的免疫调节活性的结构要求,并为将来的治疗发展提供指导原则,我们准备并评估了几种亚谷蛋白类似物的免疫调节活性。我们的努力确定了结构复杂性降低的谷胱甘肽类似物作为未来自身免疫药物开发的潜在先导化合物。
表征谱图
-
氢谱1HNMR
-
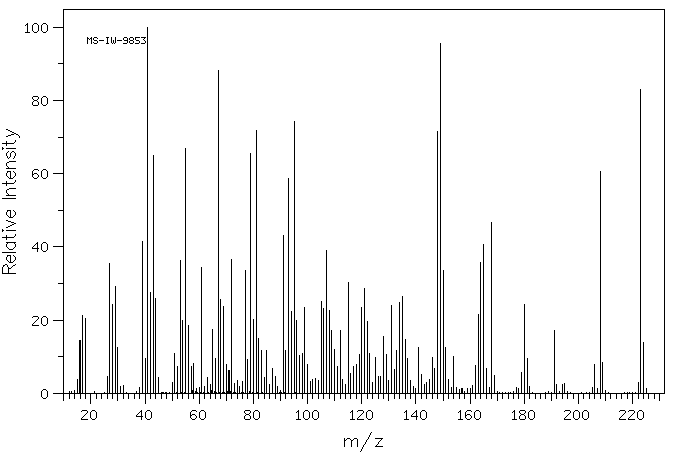
质谱MS
-
碳谱13CNMR
-
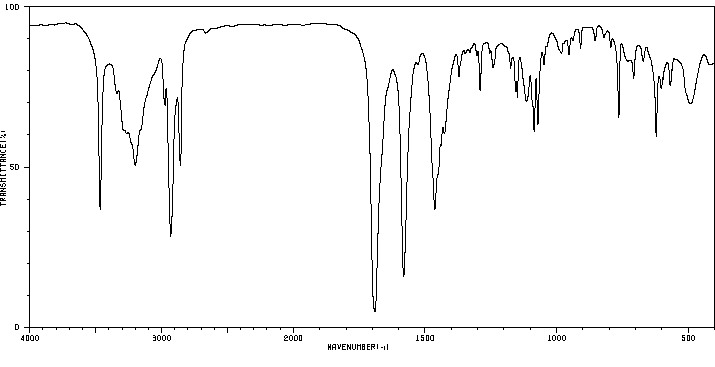
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷