脯氨酰丙氨酸 | 6422-36-2
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:232 °C
-
沸点:454.2±45.0 °C(Predicted)
-
密度:1.218±0.06 g/cm3(Predicted)
-
溶解度:水(轻微,超声处理)
计算性质
-
辛醇/水分配系数(LogP):-3.7
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:78.4
-
氢给体数:3
-
氢受体数:4
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:-15°C
SDS
Section 1. Chemical Product and Company Identification
L-Prolyl-L-alanine
Common Name/
Trade Name
Manufacturer
Commercial Name(s)
Synonym
Chemical Name
Chemical Family
L-Prolyl-L-alanine
Section 4. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at
least 15 minutes. Get medical attention if irritation occurs.
Skin Contact Wash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops.
Serious Skin Contact Not available.
Inhalation If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get
medical attention.
Serious Inhalation Not available.
Ingestion Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an
unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight
clothing such as a collar, tie, belt or waistband.
Serious Ingestion Not available.
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
Flash Points Not available.
Flammable Limits Not available.
Products of Combustion These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...).
Fire Hazards in Presence of Slightly flammable to flammable in presence of heat.
Various Substances
Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
of Various Substances
SMALL FIRE: Use DRY chemical powder.
Fire Fighting Media
and Instructions LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
As with most organic solids, fire is possible at elevated temperatures
Special Remarks on
Fire Hazards
Special Remarks on Explosion Not available.
Hazards
Section 6. Accidental Release Measures
Small Spill Use appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by
spreading water on the contaminated surface and dispose of according to local and regional authority
requirements.
Large Spill Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water
on the contaminated surface and allow to evacuate through the sanitary system.
L-Prolyl-L-alanine
Section 7. Handling and Storage
Precautions Keep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not
breathe dust. Keep away from incompatibles such as oxidizing agents, reducing agents, alkalis.
Storage Keep container tightly closed. Keep container in a cool, well-ventilated area. Do not store above 0°C (32°F).
Freeze
Section 8. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection
Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves.
Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used
a Large Spill to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist
BEFORE handling this product.
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Physical state and appearance Solid. Odor Not available.
Taste Not available.
Molecular Weight 186.21 g/mole
Color Not available.
pH (1% soln/water) Not available.
Boiling Point Not available.
Melting Point Not available.
Critical Temperature Not available.
Not available.
Specific Gravity
Vapor Pressure Not applicable.
Vapor Density Not available.
Volatility Not available.
Odor Threshold Not available.
Not available.
Water/Oil Dist. Coeff.
Ionicity (in Water) Not available.
Not available.
Dispersion Properties
Solubility Not available.
Section 10. Stability and Reactivity Data
Stability The product is stable.
Not available.
Instability Temperature
Conditions of Instability Excess heat, incompatible materials.
Incompatibility with various Reactive with oxidizing agents, reducing agents, alkalis.
substances
Corrosivity Not available.
L-Prolyl-L-alanine
Special Remarks on Also incompatible with alkali metals.
Reactivity
Special Remarks on Not available.
Corrosivity
Polymerization Will not occur.
Section 11. Toxicological Information
Routes of Entry Inhalation. Ingestion.
Toxicity to Animals LD50: Not available.
LC50: Not available.
Chronic Effects on Humans Not available.
Other Toxic Effects on Slightly hazardous in case of skin contact (irritant), of ingestion, of inhalation.
Humans
Special Remarks on Not available.
Toxicity to Animals
Special Remarks on
Not available.
Chronic Effects on Humans
Special Remarks on other Acute Potential Health Effects:
Toxic Effects on Humans Skin: May cause skin irritation.
Eyes: May cause eye irritation.
Inhalation: Dust may cause respiratory tract irritation.
Ingestion: Expected to be a low hazard.
Section 12. Ecological Information
Ecotoxicity Not available.
BOD5 and COD Not available.
Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may
arise.
Toxicity of the Products The product itself and its products of degradation are not toxic.
of Biodegradation
Special Remarks on the Not available.
Products of Biodegradation
Section 13. Disposal Considerations
Waste Disposal Waste must be disposed of in accordance with federal, state and local environmental
control regulations.
Section 14. Transport Information
DOT Classification Not a DOT controlled material (United States).
Identification Not applicable.
Special Provisions for Not applicable.
Transport
L-Prolyl-L-alanine
DOT (Pictograms)
Section 15. Other Regulatory Information and Pictograms
No products were found.
Federal and State
Regulations
California California prop. 65: This product contains the following ingredients for which the State of California has found
to cause cancer which would require a warning under the statute: No products were found.
Proposition 65
Warnings
California prop. 65: This product contains the following ingredients for which the State of California has found
to cause birth defects which would require a warning under the statute: No products were found.
Other Regulations EINECS: This product is not on the European Inventory of Existing Commercial Chemical Substances.
Canada: Not listed on Canadian Domestic Substance List (DSL) or Canadian Non- Domestic Substance List
(NDSL).
China: Not listed on National Inventory.
Japan: Not listed on National Inventory (ENCS).
Korea: Not listed on National Inventory (KECI).
Philippines: Not listed on National Inventory (PICCS).
Australia: Not listed on AICS.
Other Classifications WHMIS (Canada) Not controlled under WHMIS (Canada).
DSCL (EEC) This product is not classified according Not applicable.
to the EU regulations.
Health Hazard
HMIS (U.S.A.) 1 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
1 0 Reactivity
Health
Reactivity
0
Specific hazard
Personal Protection
E
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
TDG (Canada)
(Pictograms)
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves.
L-Prolyl-L-alanine
Lab coat.
Dust respirator. Be sure to use an
approved/certified respirator or
equivalent.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (3S,8aS)-六氢-3-甲基吡咯并[1,2-a]吡嗪-1,4-二酮 (3S,8aS)-3-methylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione 36357-32-1 C8H12N2O2 168.195
反应信息
-
作为反应物:描述:参考文献:名称:Chemical Characterization of Diketopiperazines in Beer摘要:Diketopiperazines (DKPs) corresponding to cyclic dipeptides have been detected in a variety of natural products as well as in processed foods, beverages, and food and beverage ingredients. A series of seven proline-based diketopiperazines, namely, cyclo(Ala-Pro), cyclo(Val-Pro), cyclo(Ile-Pro), cyclo(Leu-Pro), cyclo(Met-Pro), cyclo(Phe-Pro), and cyclo(Pro-Pro), has now been identified in beer. A marketplace cross-section of five commercial beers was studied, involving products manufactured in different countries using distinctly different raw materials and brewing styles; maximum concentrations of individual diketopiperazines in various beers ranged from below detection limit to approximately 24 ppm. The flavor characteristics of these compounds were described variously as bitter, mouth coating, drying, astringent, salty, metallic, and grainy when evaluated in water at concentrations ranging fi om 10 to 50 ppm. However, it is questionable whether or not the diketopiperazines reported here make a significant contribution to either the aroma or taste of most beers.DOI:10.1021/jf9700992
-
作为产物:参考文献:名称:Tomida, Ichiro; Kimura, Yoichi; Kudo, Ikuko, Agricultural and Biological Chemistry, 1985, vol. 49, # 2, p. 529 - 532摘要:DOI:
-
作为试剂:参考文献:名称:[EN] CATALYTIC ASYMMETRIC SYNTHESIS OF OPTICALLY ACTIVE alpha-HALO-CARBONYL COMPOUNDS
[FR] SYNTHESE ASYMETRIQUE CATALYTIQUE DE COMPOSES DE DOLLAR G(A)-HALO-CARBONYLE OPTIQUEMENT ACTIFS摘要:一种用于催化不对称合成光学活性化合物的方法,其化学式为(la)或(lb):其中R是有机基团;X是卤素;R1和R2可以相同也可以不同,分别代表H,或者是有机基团,或者R1和R2可以通过形成环系统的桥连接在一起;R和R2可以通过形成环系统的桥连接在一起;在R和R1不同且R2与H不同时通过碳-碳键连接时,包括以下步骤:将化合物(2)与卤化试剂在含有含有手性氮的有机化合物的催化剂量的情况下反应。公开号:WO2005080298A1
文献信息
-
Electron spin resonance of spin trapped radicals in γ-irradiated polycrystalline dipeptides. Chromatographic separation of radicals作者:Keisuke Makino、Peter RieszDOI:10.1139/v82-214日期:1982.6.15
Polycrystalline dipeptides (glycyl-glycine, glycyl-L-valine, glycyl-L-leucine, L-alanyl-glycine, and L-prolyl-L-alanine) were γ-irradiated at room temperature in the absence of air. Subsequently they were dissolved in aqueous solutions containing 2-methyl-2-nitrosopropane as the spin trap. From the esr spectra of the nitroxide radicals separated by high-performance liquid chromatography, structural assignments of the radicals were made. For glycyl peptides, H-abstraction from the α-carbon atoms of the carboxyl terminal residues and from the side-chains were observed. For L-alanyl-glycine, H-abstraction from the glycyl residue and the formation of the deamination radical could be shown to occur. For L-prolyl-L-alanine, the ring opening (deamination) reaction, decarboxylation and H-abstraction from the C-terminal a-carbon were seen.
-
Elektrochemische Decarboxylierung vonL.-Threonin- und Oligopeptid-Derivaten unter Bildung vonN-Acyl-N, O-acetalen: Herstellung von Oligopeptiden mit Carboxamid-oder Phosphonat-C-Terminus作者:Dieter Seebach、Roland Charczuk、Christian Gerber、Philippe Renaud、Heinz Berner、Helmut SchneiderDOI:10.1002/hlca.19890720302日期:1989.5.3Electrochemical Decarboxylation of L-Threonine and Oligopeptide Derivatives with Formation of N-Acyl-N, O-acetals: Preparation of Oligopeptides with Amide or Phophonate C-Terminus
-
PROCESSES FOR PREPARING JAK INHIBITORS AND RELATED INTERMEDIATE COMPOUNDS申请人:Zhou Jiacheng公开号:US20100190981A1公开(公告)日:2010-07-29The present invention is related to processes for preparing chiral substituted pyrazolyl pyrrolo[2,3-d]pyrimidines of Formula III, and related synthetic intermediate compounds. The chiral substituted pyrazolyl pyrrolo[2,3-d]pyrimidines are useful as inhibitors of the Janus Kinase family of protein tyrosine kinases (JAKs) for treatment of inflammatory diseases, myeloproliferative disorders, and other diseases.
-
[EN] PROCESSES FOR PREPARING JAK INHIBITORS AND RELATED INTERMEDIATE COMPOUNDS<br/>[FR] PROCÉDÉS DE PRÉPARATION D'INHIBITEURS DES JAK ET COMPOSÉS INTERMÉDIAIRES APPARENTÉS申请人:INCYTE CORP公开号:WO2010083283A2公开(公告)日:2010-07-22The present invention is related to processes for preparing chiral substituted pyrazolyl pyrrolo[2,3-di]pyrimidines of Formula III, and related synthetic intermediate compounds. The chiral substituted pyrazolyl pyrrolo[2,3-d]pyrimidines are useful as inhibitors of the Janus Kinase family of protein tyrosine kinases (JAKs) for treatment of inflammatory diseases, myeloproliferative disorders, and other diseases.
-
Aryloxy and arylacyloxy methyl ketones as thiol protease inhibitors申请人:Syntex (U.S.A.) Inc.公开号:US05158936A1公开(公告)日:1992-10-27Thiol protease inhibitors are disclosed having the formula: ##STR1## or an optical isomer thereof, or a pharmaceutically acceptable salt thereof, wherein: n is 0 or 1; m is 0, 1 or 2; X is H or an N-protecting group; each Y is independently an optionally protected .alpha.-amino acid residue; R is an optionally protected .alpha.-amino acid side chain that is H or CH.sub.3 or that is bonded to the .alpha.-carbon atom to which it is attached by a methylene, methine or phenyl radial; and R' is optionally substituted aryl.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
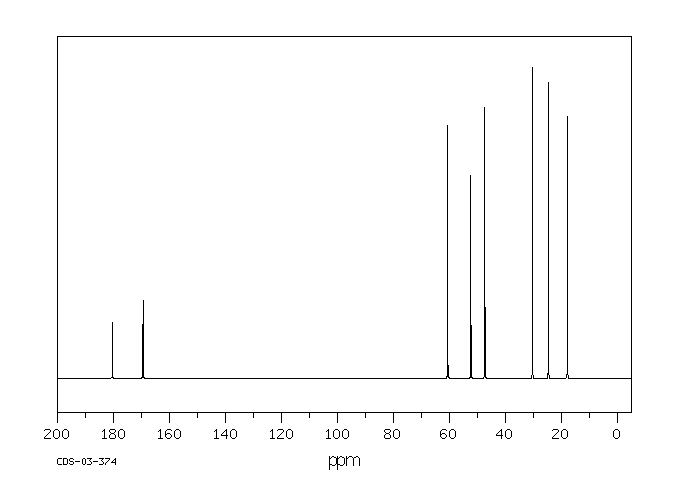
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息