6-氨基-2-甲基-2-庚醇 | 372-66-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:<25 °C
-
沸点:115 °C (11.2515 mmHg)
-
密度:0.895
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
保留指数:1101.1;1118
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:46.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险品标志:C
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R34
-
海关编码:2922199090
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | 6-Amino-2-methyl-2-heptanol 98% Material Safety Data Sheet |
| Synonym: | Heptaminol; 2-Methyl-2-hydroxy-6-aminoheptan |
| CAS: | 372-66-7 |
Synonym:Heptaminol; 2-Methyl-2-hydroxy-6-aminoheptan
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 372-66-7 | 6-Amino-2-methyl-2-heptanol | 98.0 | 206-758-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 372-66-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: oily, colorless
Odor: amine-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 92 - 93 deg C @ 7.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: miscible
Specific Gravity/Density: .8950g/cm3
Molecular Formula: C8H19NO
Molecular Weight:
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stability unknown.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 372-66-7: MJ3230000 LD50/LC50:
Not available.
Carcinogenicity:
6-Amino-2-methyl-2-heptanol - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 372-66-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 372-66-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 372-66-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (R)-6-amino-2-methylheptan-2-ol 165962-54-9 C8H19NO 145.245 1,5-二甲基己胺(2-氨基-6-甲基庚烷) 1,5-dimethylhexylamine 543-82-8 C8H19N 129.246 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-6-amino-2-methylheptan-2-ol 165962-55-0 C8H19NO 145.245 —— (R)-6-amino-2-methylheptan-2-ol 165962-54-9 C8H19NO 145.245 —— 2-acetamido-6-hydroxy-6-methyl-heptane 85602-77-3 C10H21NO2 187.282
反应信息
-
作为反应物:描述:参考文献:名称:Resorcinol compounds摘要:具有以下公式的新化合物: 其中W为氢、卤素、n-烷基、-NHCOR.sup.1、-COR.sup.1或苯氧乙酰氨基,可选地被一个或两个C.sub.1-12直链或支链烷基取代,X为偶联位置的取代基,可选自氢、氯、溴、-SR.sup.11或连接在环氮原子上的含氮杂环残基,Y为具有以下公式的基团: 其中Q选自残基:-COOR.sup.4或-CONR.sup.4R.sup.5、-OM、-NR.sup.7R.sup.8、-PO(OR.sup.9)[O].sub.xR.sup.10(其中X=0或1)、-SO.sub.2OH、-SO.sub.2NR.sup.4R.sup.5或CN。这些化合物被用作摄影材料中的黑色显色剂。R.sup.1、R.sup.2、R.sup.3、R.sup.4、R.sup.5、R.sup.7、R.sup.8、R.sup.9、R.sup.10、R.sup.11、M、k和n在此后定义。公开号:US04661601A1
-
作为产物:描述:(R)-6-amino-2-methylheptan-2-ol 在 2,2,2-三氟乙硫醇 作用下, 以 四氢呋喃 、 3-甲基-3-戊醇 为溶剂, 反应 2.0h, 生成 6-氨基-2-甲基-2-庚醇参考文献:名称:En Route to (S)-Selective Chemoenzymatic Dynamic Kinetic Resolution of Aliphatic Amines. One-Pot KR/Racemization/KR Sequence Leading to (S)-Amides摘要:A one-pot sequential process, involving a radical racemization and an enzymatic resolution, provides access to (S)-amides, from racemic amines, with ee and yields ranging from 78 to 94% and 58 to 80%, respectively.DOI:10.1021/jo900074w
-
作为试剂:描述:、 盐酸 、 苯胺 、 盐酸庚胺醇 、 、 sodium hydroxide 在 氯化锌 乙醚 、 水 、 苯胺 、 6-氨基-2-甲基-2-庚醇 作用下, 以 水 为溶剂, 反应 18.0h, 以there was obtained 18 parts of 2-amino-6-(4-aminophenyl)-6-methylheptane b12 192°-199° C. (82% yield based on heptaminol) with the following percentage composition by weight的产率得到参考文献:名称:New diamines and a process for their production摘要:具有以下公式的新氨基烷基苯胺:##STR1## 其中k为0或1;QNH.sub.2是公式##STR2##的残基,其位于苯环上的氨基基团的正交或对位,并且n是1到15的整数,R.sub.1是C.sub.1-C.sub.8烷基,R.sub.2是C.sub.1-C.sub.4烷基或R.sub.1和R.sub.2与它们所附着的碳原子一起形成C.sub.5-C.sub.8环烷基残基,R.sub.3是H或C.sub.1-C.sub.6烷基,C.sub.3-C.sub.8环烷基或C.sub.6-C.sub.10芳基;R.sub.4和R.sub.5是H或C.sub.1-C.sub.4烷基;以及具有有机或无机酸和金属盐络合物的公式I化合物的相应盐;它们的制备过程;以及它们作为聚酰胺的中间体的用途。公开号:US04647699A1
文献信息
-
Cyclohexylamine oxidase as a useful biocatalyst for the kinetic resolution and dereacemization of amines作者:Hannes Leisch、Stephan Grosse、Hiroaki Iwaki、Yoshie Hasegawa、Peter C.K. LauDOI:10.1139/v11-086日期:2012.1
The biocatalytic performance of a cloned cyclohexylamine oxidase derived from Brevibacterium oxydans IH-35A towards structurally different amines was investigated. Cycloalkyl primary amines, alkyl aryl amines, and α-carbon-substituted aliphatic amines were identified as suitable substrates for the biocatalyst based on an activity assay. Kinetic resolutions of several amines by either recombinant whole cells or crude enzyme extracts prepared therefrom gave enantiomerically pure (R)-amines besides the corresponding ketones. When cyclohexylamine oxidase in combination with a borane–ammonia complex as reducing agent was applied to the deracemization of several substrates, excellent enantiomeric ratios (>99:1) and good isolated yields (62%–75%) of the corresponding (R)-amines were obtained.
-
[EN] COMPOSITIONS OF SELENOORGANIC COMPOUNDS AND METHODS OF USE THEREOF<br/>[FR] COMPOSITIONS DE COMPOSÉS SÉLÉNOORGANIQUES ET LEURS PROCÉDÉS D'UTILISATION CORRESPONDANTS申请人:ALLTECH INC公开号:WO2015137983A1公开(公告)日:2015-09-17The present application relates to compositions comprising selenium compounds, such as 5'-Methylselenoadenosine, Se-Adenosyl-L -homocysteine, Gamma- glutamyl-methylseleno-cysteine, a compound of formula (I), formula (II), a compound of formula (III) and combinations thereof, and methods of using the same in enhancing mitochondrial function, or treating mitochondrial dysfunction.
-
Methods and compositions for treating amyloid-related diseases申请人:Kong Xianqi公开号:US20060223855A1公开(公告)日:2006-10-05Methods, compounds, pharmaceutical compositions and kits are described for treating or preventing amyloid-related disease.描述了用于治疗或预防与淀粉样蛋白相关疾病的方法、化合物、药物组合物和试剂盒。
-
[DE] VERFAHREN ZUR HERSTELLUNG VON QUARTÄREN AMMONIUM-VERBINDUNGEN<br/>[EN] METHOD FOR PRODUCING QUATERNARY AMMONIUM COMPOUNDS<br/>[FR] PROCÉDÉ DE PRODUCTION DE COMPOSÉS D'AMMONIUM QUATERNAIRES申请人:BASF AG公开号:WO2005115969A1公开(公告)日:2005-12-08Die vorliegende Erfindung betrifft ein Verfahren zur Herstellung von quartären Ammonium-Verbindungen, bei dem man Verbindungen, die ein sp3-hybridisiertes Stickstoffatom enthalten, mit einem Dialkylsulfat oder Trialkylphosphat umsetzt und die so erhaltene Ammonium-Verbindung einem Anionenaustausch unterzieht.
-
Hydroxylated nebivolol metabolites申请人:O'Donnell P. John公开号:US20070014733A1公开(公告)日:2007-01-18Hydroxylated nebivolol metabolites increase NO release from human endothelial cell preparations in a concentration dependent fashion following acute administration. In addition, hydroxylated nebivolol metabolites, including but not limited to 4-hydroxy-6,6′difluoro-, 4-hydroxy-5-phenol-6,6′difluoro-, and 4-hydroxy-8-pheno-6,6′difluoro-, have the ability to increase the capacity for NO release in human endothelial cells following chronic administration. This invention provides hydroxylated nebivolol metabolites and compositions comprising nebivolol and/or at least one hydroxylated metabolite of nebivolol and/or at least one additional compound used to treat cardiovascular diseases or a pharmaceutically acceptable salt thereof. In addition, this invention provides methods of treating and/or preventing vascular diseases by administering at least one hydroxylated metabolite of nebivolol that is capable of releasing a therapeutically effective amount of nitric oxide to a targeted site affected by the vascular disease. Also, this invention is directed to the treatment and/or prevention of migraine headaches administering at least one hydroxylated metabolite of nebivolol. This invention may also be used in conjunction with or as a single treatment of metabolic syndrome disorders.羟基化奈必洛尔代谢物在急性给药后以浓度依赖性方式增加人内皮细胞制剂的一氧化氮释放。此外,羟基化奈必洛尔代谢物,包括但不限于4-羟基-6,6'-二氟代-、4-羟基-5-苯酚-6,6'-二氟代-和4-羟基-8-苯并-6,6'-二氟代-,在慢性给药后能够增加人内皮细胞的一氧化氮释放能力。本发明提供了羟基化奈必洛尔代谢物和包含奈必洛尔和/或至少一种羟基化奈必洛尔代谢物和/或至少一种用于治疗心血管疾病的附加化合物的组合物,以及可药用的盐。此外,本发明还提供了通过给药至少一种能够释放治疗有效量的一氧化氮到受血管疾病影响的靶向部位的羟基化奈必洛尔代谢物来治疗和/或预防血管疾病的方法。本发明还涉及通过给药至少一种羟基化奈必洛尔代谢物来治疗和/或预防偏头痛。本发明还可以与治疗代谢综合征障碍的其他治疗联合使用,或作为单一治疗。
表征谱图
-
氢谱1HNMR
-
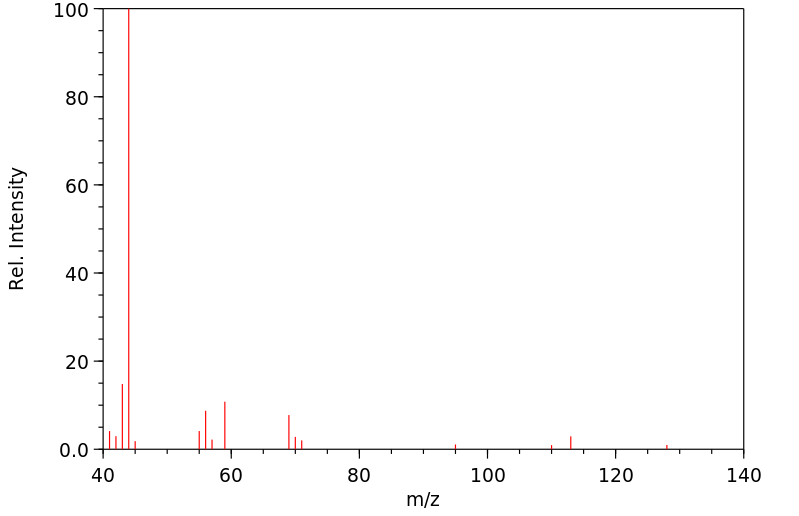
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息