6-氨基苯并噻唑 | 533-30-2
中文名称
6-氨基苯并噻唑
中文别名
6-氨基苯并[D]噻唑;1,3-苯并噻唑-6-胺;6-氨基苯并噻吩盐酸盐
英文名称
1,3-benzothiazol-6-amine
英文别名
6-amino-benzothiazole;benzo[d]thiazol-6-amine;benzothiazol-6-ylamine;benzothiazole-6-amine;1,3-benzothiazole-6-amine;benzothiazol-6-amine;6-Aminobenzothiazole
CAS
533-30-2
化学式
C7H6N2S
mdl
——
分子量
150.204
InChiKey
FAYAYUOZWYJNBD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:87-91 °C (lit.)
-
沸点:323.1±15.0 °C(Predicted)
-
密度:1.2162 (rough estimate)
-
溶解度:可溶于氯仿、甲醇(少许)
-
稳定性/保质期:
请按照规定使用和储存,以免发生分解,并避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S22,S26,S36,S36/37/39
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:2934999090
-
危险品标志:Xn
-
危险类别码:R20/21/22,R36/37/38
-
RTECS号:DL1050200
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:储存于阴凉、干燥、通风良好的库房中。远离火种和热源,避免阳光直射,并确保包装密封。与酸类及食用化学品分开存放,切忌混储。储区应备有合适的材料以处理可能的泄漏。
SDS
| Name: | 1,3-Benzothiazol-6-amine Material Safety Data Sheet |
| Synonym: | None Known. |
| CAS: | 533-30-2 |
Synonym: None Known.
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 533-30-2 | 1,3-Benzothiazol-6-amine | 97+% | 208-559-4 |
Risk Phrases: 20/21/22 36/37/38
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Harmful by inhalation, in contact with skin and if swallowed. Irritating to eyes, respiratory system and skin. Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. Causes digestive tract irritation.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
No information found.
SECTION 4 - FIRST AID MEASURES
Eyes:
Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation.
SECTION 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 533-30-2: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Orange
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 98.5 - 99.5 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H6N2S
Molecular Weight: 150.2
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable.
Conditions to Avoid:
Incompatible materials, dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids, reducing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 533-30-2: DL1050200
LD50/LC50:
Not available.
Carcinogenicity:
1,3-Benzothiazol-6-amine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with skin and if swallowed. R 36/37/38 Irritating to eyes, respiratory system and skin.
Safety Phrases:
S 22 Do not breathe dust. S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 Wear suitable protective clothing, gloves and eye/face protection. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). WGK (Water Danger/Protection) CAS# 533-30-2: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 533-30-2 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 533-30-2 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 10/08/2004 Revision #1 Date: 12/17/2004 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-硝基苯并噻唑 6-nitrobenzo[d]thiazole 2942-06-5 C7H4N2O2S 180.187 苯并噻唑 1,3-Benzothiazole 95-16-9 C7H5NS 135.189 2-氨基-6-硝基苯并噻唑 2-amino-6-nitrobenzothiazole 6285-57-0 C7H5N3O2S 195.202 2-氯-6-硝基苯并噻唑 2-chloro-6-nitrobenzothiazole 2407-11-6 C7H3ClN2O2S 214.632 2-氟-6-硝基-1,3-苯并噻唑 2-fluoro-6-nitro-benzothiazole 1131-75-5 C7H3FN2O2S 198.177 6-硝基-2-苯并噻唑啉酮 6-nitro-2(3H)-benzothiazolone 28620-12-4 C7H4N2O3S 196.186 6-溴苯并噻唑 6-bromo-1,3-benzothiazole 53218-26-1 C7H4BrNS 214.085 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-甲基-6-苯并噻唑胺 6-(N-methylamino)benzothiazole 161557-60-4 C8H8N2S 164.231 噻唑,6-肼- 6-hydrazino-1,3-benzothiazole 474123-23-4 C7H7N3S 165.219 —— N,N-dimethyl-1,3-benzothiazol-6-amine 79138-22-0 C9H10N2S 178.258 —— 6-benzothiazolylisocyanate 141492-51-5 C8H4N2OS 176.199 —— 6-phenylamino-1,3-benzothiazole —— C13H10N2S 226.302 N-6-苯并噻唑基-乙酰胺 N-acetyl-6-aminobenzothiazole 58249-63-1 C9H8N2OS 192.241 —— 1-(benzothiazol-6-ylamino)-butan-2-one 1260009-39-9 C11H12N2OS 220.295 —— 1,3-di(benzo[d]thiazol-6-yl)thiourea 1360449-06-4 C15H10N4S3 342.469 —— N-phenyl-N'-benzothiazol-6-ylurea 343613-71-8 C14H11N3OS 269.327 —— 1,3-di(benzo[d]thiazol-6-yl)urea 1360448-95-8 C15H10N4OS2 326.403 —— N-(benzo[d]thiazol-6-yl)propionamide 922920-21-6 C10H10N2OS 206.268 —— ethyl 2-(benzo[d]thiazol-6-ylamino)acetate —— C11H12N2O2S 236.294 —— N-(benzo[d]thiazol-6-yl)methanesulfonamide 1000312-89-9 C8H8N2O2S2 228.296 —— N-benzothiazol-6-yl-2-hydroxyimino-acetamide —— C9H7N3O2S 221.239 6-三氟乙酰氨基-苯并噻唑 6-trifluoroacetamido-benzothiazole 161557-59-1 C9H5F3N2OS 246.213 —— N-methyl-N-(benzothiazol-6-yl)carbamoyl chloride 220744-18-3 C9H7ClN2OS 226.686 —— 6-N-(2,4-diaminoanilino)benzthiazole 77464-30-3 C13H12N4S 256.331 7-碘苯并[d]噻唑-6-胺 6-amino-7-iodo-1,3-benzothiazole 914366-54-4 C7H5IN2S 276.101 —— benzothiazol-6-yl-thiophen-2-ylmethylamine 1592896-26-8 C12H10N2S2 246.357 6-氨基-7-溴苯并噻唑 6-amino-7-bromobenzothiazole 769-20-0 C7H5BrN2S 229.1 —— 7-chloro-benzothiazol-6-ylamine 70202-00-5 C7H5ClN2S 184.649 —— 6-(tert-butoxycarbonylamino)benzo[d]thiazole 1192841-91-0 C12H14N2O2S 250.321 —— N-benzothiazol-6-yl-benzamide 135249-30-8 C14H10N2OS 254.312 6-碘苯并[d]噻唑 6-iodobenzothiazole 654070-00-5 C7H4INS 261.086 —— 6-amino-2-butylthiobenzothiazole 73844-23-2 C11H14N2S2 238.378 —— benzothiazol-6-yl-(4,6-dichloro-[1,3,5]-triazin-2-yl)amine 797039-38-4 C10H5Cl2N5S 298.155 —— 6-(2-Thiazolin-2-ylamino)benzothiazole 196204-81-6 C10H9N3S2 235.334 6-羟基苯并噻唑 6-hydroxybenzothiazole 13599-84-3 C7H5NOS 151.189 —— phenyl benzo[d]thiazol-6-ylcarbamate —— C14H10N2O2S 270.312 6-溴苯并噻唑 6-bromo-1,3-benzothiazole 53218-26-1 C7H4BrNS 214.085 —— N-Benzothiazol-6-yl-N-methyl-methanesulfonamide 1000312-90-2 C9H10N2O2S2 242.323 —— N-(6-chloropyridazin-3-yl)-1,3-benzothiazol-6-amine 1032184-69-2 C11H7ClN4S 262.722 2-苯基-苯并噻唑-6-胺 6-Amino-2-phenyl-benzothiazol 6392-97-8 C13H10N2S 226.302 —— (benzothiazol-6-ylamino-methylene)-malonic acid diethyl ester 33109-74-9 C15H16N2O4S 320.369 7-溴苯并噻唑 7-bromobenzo[d]thiazole 767-70-4 C7H4BrNS 214.085 —— 2-(p-tolyl)benzo[d]thiazol-6-amine 53544-76-6 C14H12N2S 240.329 —— N-(benzothiazol-6-yl)-2-(3-methoxyphenyl)acetamide 1213782-67-2 C16H14N2O2S 298.365 1,3-苯并噻唑-6-基硼酸 benzo[d]thiazol-6-ylboronic acid 499769-91-4 C7H6BNO2S 179.007 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:6-氨基苯并噻唑 在 2,2,6,6-四甲基哌啶 作用下, 以 乙醇 、 水 为溶剂, 90.0 ℃ 、1.03 MPa 条件下, 反应 4.25h, 生成 (Z)-5-(2-(1H-indol-3-yl)-2-oxoethylidene)-3-(benzo[d]thiazol-6-yl)-2-thioxothiazolidin-4-one参考文献:名称:一种有效的微波辅助合成新型罗丹宁衍生物作为潜在的 HIV-1 和 JSP-1 抑制剂摘要:( Z )-5-(2-(1 H -Indol-3-yl)-2-oxoethylidene)-3-phenyl-2-thioxothiazolidin-4-one ( 7a – q ) 衍生物已通过缩合反应合成3-苯基-2-硫代噻唑烷-4-酮(3a - h)与适当取代的2-(1H-吲哚-3-基)-2-氧代乙醛(6a - d)在微波条件下。thioxothiazolidine-4-ones 是由相应的芳香胺 ( 1a - e ) 和二-(羧甲基)-三硫代羰基 ( 2 ) 制备的。醛 ( 6a – h)使用 HSnBu 3由相应的酰氯 ( 5a – d ) 合成。DOI:10.1016/j.tetlet.2011.05.114
-
作为产物:描述:参考文献:名称:Nishizawa, Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1942, vol. 62, p. 47,51; dtsch. Ref. S. 19, 22摘要:DOI:
文献信息
-
IRAK DEGRADERS AND USES THEREOF申请人:Kymera Therapeutics, Inc.公开号:US20190192668A1公开(公告)日:2019-06-27The present invention provides compounds, compositions thereof, and methods of using the same.本发明提供了化合物、其组合物以及使用这些化合物的方法。
-
Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications作者:Lixing Zhao、Chenyang Hu、Xuefeng Cong、Gongda Deng、Liu Leo Liu、Meiming Luo、Xiaoming ZengDOI:10.1021/jacs.0c12318日期:2021.1.27Transition metal catalysis that utilizes N-heterocyclic carbenes as noninnocent ligands in promoting transformations has not been well studied. We report here a cyclic (alkyl)(amino)carbene (CAAC) ligand-promoted nitro deoxygenative hydroboration with cost-effective chromium catalysis. Using 1 mol % of CAAC-Cr precatalyst, the addition of HBpin to nitro scaffolds leads to deoxygenation, allowing for利用 N-杂环卡宾作为非无害配体促进转化的过渡金属催化尚未得到很好的研究。我们在这里报告了具有成本效益的铬催化的环状(烷基)(氨基)卡宾(CAAC)配体促进的硝基脱氧硼氢化反应。使用 1 mol % 的 CAAC-Cr 预催化剂,将 HBpin 添加到硝基支架上会导致脱氧,从而保留各种可还原的官能团和敏感基团对硼氢化的相容性,从而提供一种温和、化学选择性和易于形成的策略苯胺,以及杂芳基和脂肪胺衍生物,具有广泛的范围和特别高的转换数(高达 1.8 × 106)。基于理论计算的机械研究,表明CAAC配体在促进HBpin氢化物极性反转中起重要作用;它用作 H 穿梭以促进脱氧硼氢化。通过这种策略制备的几种市售药物突出了其在药物化学中的潜在应用。
-
[EN] CYCLIC SULFAMIDE COMPOUNDS AND METHODS OF USING SAME<br/>[FR] COMPOSÉS DE SULFAMIDE CYCLIQUE ET LEURS PROCÉDÉS D'UTILISATION申请人:ASSEMBLY BIOSCIENCES INC公开号:WO2018160878A1公开(公告)日:2018-09-07The present disclosure provides, in part, cyclic sulfamide compounds, and pharmaceutical compositions thereof, useful as modulators of Hepatitis B (HBV) core protein, and methods of treating Hepatitis B (HBV) infection.本公开提供了部分环磺胺化合物及其药物组合物,可用作乙型肝炎(HBV)核心蛋白的调节剂,并用于治疗乙型肝炎(HBV)感染的方法。
-
Synthesis of N- aryl and N -heteroaryl hydroxylamines via partial reduction of nitroarenes with soluble nanoparticle catalysts作者:Jefferson H. Tyler、S. Hadi Nazari、Robert H. Patterson、Venkatareddy Udumula、Stacey J. Smith、David J. MichaelisDOI:10.1016/j.tetlet.2016.11.105日期:2017.1ruthenium nanoparticles enable the selective hydrazine-mediated reduction of nitroarenes to hydroxylamine products in high yield and selectivity. Key to obtaining the hydroxylamine product in good yield was the use of organic solvents capable of solubilizing the polystyrene-supported nanoparticle catalyst. N-aryl and N-heteroaryl hydroxylamines are generated under exceptionally mild conditions and in
-
Metal-free chemoselective reduction of nitroaromatics to anilines via hydrogen transfer strategy作者:Qi Shuai、Jun Li、Feng Zhao、Weike Su、Guojun DengDOI:10.1007/s11696-018-0634-0日期:2019.4A novel protocol for chemoselective reduction of aromatic nitro compounds to aromatic amines has been established. The metal-free reduction goes through a hydrogen transfer process. Various easily reducible functional groups can be well tolerated under the optimized reaction conditions.
表征谱图
-
氢谱1HNMR
-
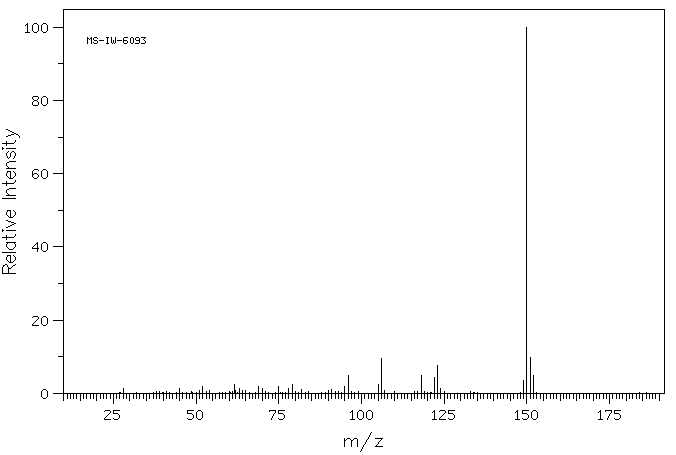
质谱MS
-
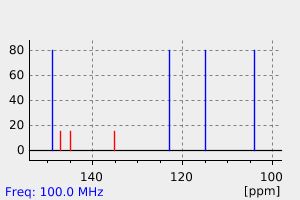
碳谱13CNMR
-
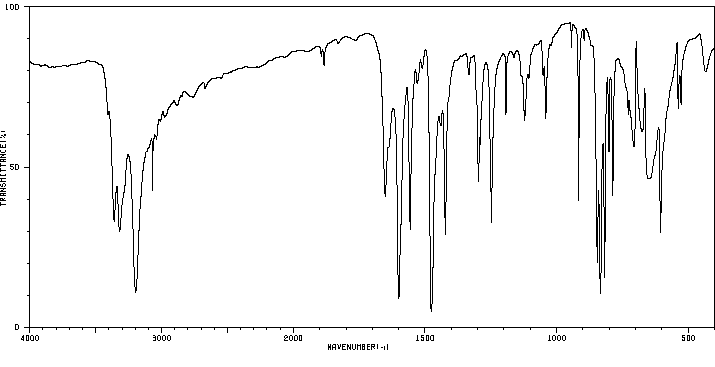
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z)-1-(3-乙基-5-羟基-2(3H)-苯并噻唑基)-2-丙酮
齐拉西酮砜
齐帕西酮-d8
阳离子蓝NBLH
阳离子荧光黄4GL
锂2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
铜酸盐(4-),[2-[2-[[2-[3-[[4-氯-6-[乙基[4-[[2-(硫代氧代)乙基]磺酰]苯基]氨基]-1,3,5-三嗪-2-基]氨基]-2-(羟基-kO)-5-硫代苯基]二氮烯基-kN2]苯基甲基]二氮烯基-kN1]-4-硫代苯酸根(6-)-kO]-,(1:4)氢,(SP-4-3)-
铜羟基氟化物
钾2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
钠3-(2-{(Z)-[3-(3-磺酸丙基)-1,3-苯并噻唑-2(3H)-亚基]甲基}[1]苯并噻吩并[2,3-d][1,3]噻唑-3-鎓-3-基)-1-丙烷磺酸酯
邻氯苯骈噻唑酮
西贝奈迪
螺[3H-1,3-苯并噻唑-2,1'-环戊烷]
螺[3H-1,3-苯并噻唑-2,1'-环己烷]
葡萄属英A
草酸;N-[1-[4-(2-苯基乙基)哌嗪-1-基]丙-2-基]-2-丙-2-基氧基-1,3-苯并噻唑-6-胺
苯酰胺,N-2-苯并噻唑基-4-(苯基甲氧基)-
苯酚,3-[[2-(三苯代甲基)-2H-四唑-5-基]甲基]-
苯胺,N-(3-苯基-2(3H)-苯并噻唑亚基)-
苯碳杂氧杂脒,N-1,2-苯并异噻唑-3-基-
苯甲酸,4-(6-辛基-2-苯并噻唑基)-
苯甲基2-甲基哌啶-1,2-二羧酸酯
苯并噻唑正离子,2-[3-(1,3-二氢-1,3,3-三甲基-2H-吲哚-2-亚基)-1-丙烯-1-基]-3-乙基-,碘化(1:1)
苯并噻唑正离子,2-[2-[4-(二甲氨基)苯基]乙烯基]-3-乙基-6-甲基-,碘化
苯并噻唑正离子,2-[(2-乙氧基-2-羰基乙基)硫代]-3-甲基-,溴化
苯并噻唑啉
苯并噻唑三氯金(III)
苯并噻唑-d4
苯并噻唑-7-乙酸
苯并噻唑-6-腈
苯并噻唑-5-羧酸
苯并噻唑-5-硼酸频哪醇酯
苯并噻唑-4-醛
苯并噻唑-4-乙酸
苯并噻唑-2-磺酸钠
苯并噻唑-2-磺酸
苯并噻唑-2-磺酰氟
苯并噻唑-2-甲醛
苯并噻唑-2-甲酸
苯并噻唑-2-甲基甲胺
苯并噻唑-2-基磺酰氯
苯并噻唑-2-基甲基-乙基-胺
苯并噻唑-2-基叠氮化物
苯并噻唑-2-基-邻甲苯-胺
苯并噻唑-2-基-己基-胺
苯并噻唑-2-基-(4-氯-苯基)-胺
苯并噻唑-2-基-(4-氟-苯基)-胺
苯并噻唑-2-基-(4-乙氧基-苯基)-胺
苯并噻唑-2-基-(2-甲氧基-苯基)-胺
苯并噻唑-2-基-(2,6-二甲基-苯基)-胺