3-butyloxirane-2-carbaldehyde | 58936-30-4
中文名称
——
中文别名
——
英文名称
3-butyloxirane-2-carbaldehyde
英文别名
3-butyl-2-oxiranecarboxaldehyde;3-butyloxirane-2-carboxaldehyde;2,3-epoxy-1-heptanal;2(RS),3(SR)-epoxyheptanal
CAS
58936-30-4
化学式
C7H12O2
mdl
MFCD08457870
分子量
128.171
InChiKey
JSGLRFSEKUNPRD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
LogP:0.95
-
物理描述:Colourless to pale yellow liquid; fatty citrus aroma
-
溶解度:Practically insoluble to insoluble in water
-
密度:0.938-0.948 (20º)
-
折光率:1.427-1.437
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:9
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.857
-
拓扑面积:29.6
-
氢给体数:0
-
氢受体数:2
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-hydroxyheptanal 21582-33-2 C7H14O2 130.187
反应信息
-
作为反应物:描述:3-butyloxirane-2-carbaldehyde 在 溶剂黄146 、 sodium phenylseleno(triethyl)borate 作用下, 以 乙醇 为溶剂, 反应 0.17h, 以85%的产率得到3-hydroxyheptanal参考文献:名称:有机硒介导的α,β-环氧酮,α,β-环氧酯及其同系物还原为β-羟基羰基化合物:合成羟醛及其类似物的新方法摘要:描述了通过使用有机硒试剂将α,β-环氧酮,α,β-环氧酯(缩水甘油酯)及其同类物还原为β-羟基羰基化合物(醛醇)的新方法。通过在EtOH中用NaBH 4还原(PhSe)2和原位生成的苯硒酚(PhSeH),可以轻松制备苯基硒基(三乙基)硼酸钠复合物Na [PhSeB(OEt)3 ]通过添加乙酸从硼酸盐配合物中得到的氯已被证明可作为这些转化的极好的还原剂。有机硒介导的α,β-环氧羰基化合物在α-碳上区域特异性还原,从而以优异的收率生产出多种环状(分子内)醇醛和非环状(分子间)醇醛。定量机理研究表明,与常见的电子转移还原剂相比,有机硒介导的还原通过α取代过程进行。DOI:10.1016/s0040-4020(97)00781-3
-
作为产物:参考文献:名称:四氧化碳和超氧化物原位生成的过氧中间体使烯烃环氧化。摘要:DOI:10.1021/ja00263a048
文献信息
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
Amine-Catalyzed Asymmetric Epoxidation of α,β-Unsaturated Aldehydes作者:Gui-Ling Zhao、Ismail Ibrahem、Henrik Sundén、Armando CórdovaDOI:10.1002/adsc.200600529日期:2007.5.7The direct organocatalytic enantioselective epoxidation of α,β-unsaturated aldehydes with different peroxides is presented. Proline, chiral pyrrolidine derivatives, and amino acid-derived imidazolidinones catalyze the asymmetric epoxidation of α,β-unsaturated aldehydes. In particular, protected commercially available α,α-diphenyl- and α,α-di(β-naphthyl)-2-prolinols catalyze the asymmetric epoxidation提出了α,β-不饱和醛与不同过氧化物的直接有机催化对映选择性环氧化反应。脯氨酸,手性吡咯烷衍生物和氨基酸衍生的咪唑烷酮可催化α,β-不饱和醛的不对称环氧化。特别是,受保护的可商购的α,α-二苯基-和α,α-二(β-萘基)-2-脯氨醇催化具有高非对映和对映选择性的α,β-不饱和醛的不对称环氧化反应,以提供相应的2 -环氧醛,收率高达97:3 dr和ee达98%。使用无毒的催化剂,水和过氧化氢,氢过氧化脲或过碳酸钠作为氧气源可以使该反应对环境无害。另外,描述了一锅直接有机催化不对称串联环氧化-Wittig反应。该反应是高度非对映体和对映体选择性的,并提供了对2,4-二环氧丁醛的快速反应。此外,提出了一种高度立体选择性的一锅法有机催化不对称级联环氧化-曼尼希反应,该反应通过亚胺和烯胺活化的结合而进行。还讨论了氨基酸和手性吡咯烷催化α,β-不饱和醛直接不对称环氧化的机理和立体化学。
-
COMPOUNDS USEFUL AS MODULATORS OF TRPM8申请人:Senomyx, Inc.公开号:US20170096418A1公开(公告)日:2017-04-06The present disclosure relates to compounds which are useful as cooling sensation compounds.本公开涉及作为冷感化合物有用的化合物。
-
Chiral azetidinone epoxides申请人:THE PRESIDENT AND FELLOWS OF HARVARD COLLEGE公开号:EP0334593A1公开(公告)日:1989-09-27Epoxyimines, formed with epoxyaldehydes, react via cycloaddition with amino-protected glycyl halides to provide 3-protected-amino-4-(substituted oxiranyl)azetidinones. Chiral epoxyimines from chiral epoxyaldehydes induce high levels of asymmetric induction during the cycloaddition to provide substantially one diastereomer of the 3,4-disubstituted azetidinone. For example, the epoxyimine formed with (2R,3S)-2-formyl-3-phenyloxirane and 2,4-dimethoxybenzylamine is reacted with phthalimidoacetyl chloride to provide [3R,4R,4(2S,3S)]-1-(2,4-dimethoxybenzyl)-3-phthalimido-4-(3-phenyloxiran-2-yl)-2-azetidinone. The epoxy-substituted azetidinones are useful intermediates for β-lactam antibiotic compounds.
-
Compounds useful as modulators of TRPM8申请人:Senomyx, Inc.公开号:US10392371B2公开(公告)日:2019-08-27Provided herein are compounds which are useful as cooling sensation compounds.本文提供的化合物可用作冷感化合物。
表征谱图
-
氢谱1HNMR
-
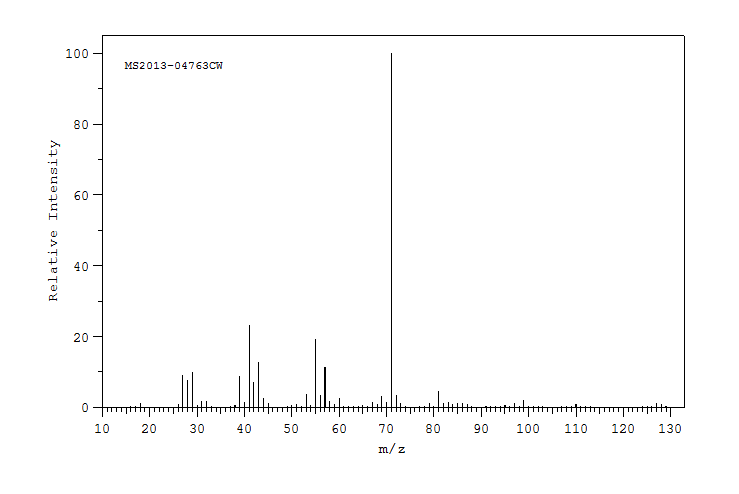
质谱MS
-
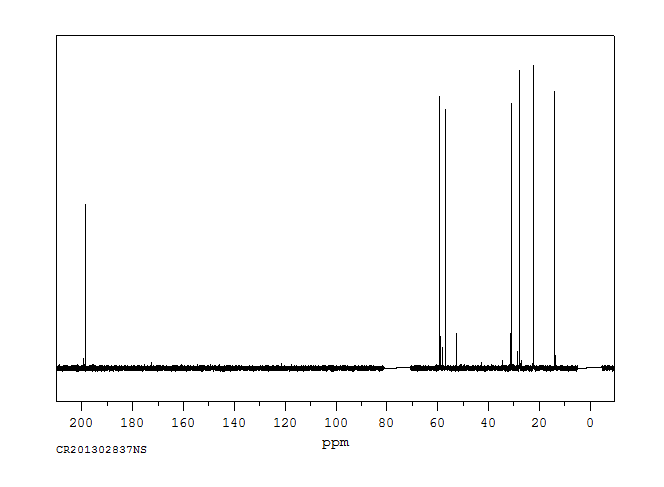
碳谱13CNMR
-
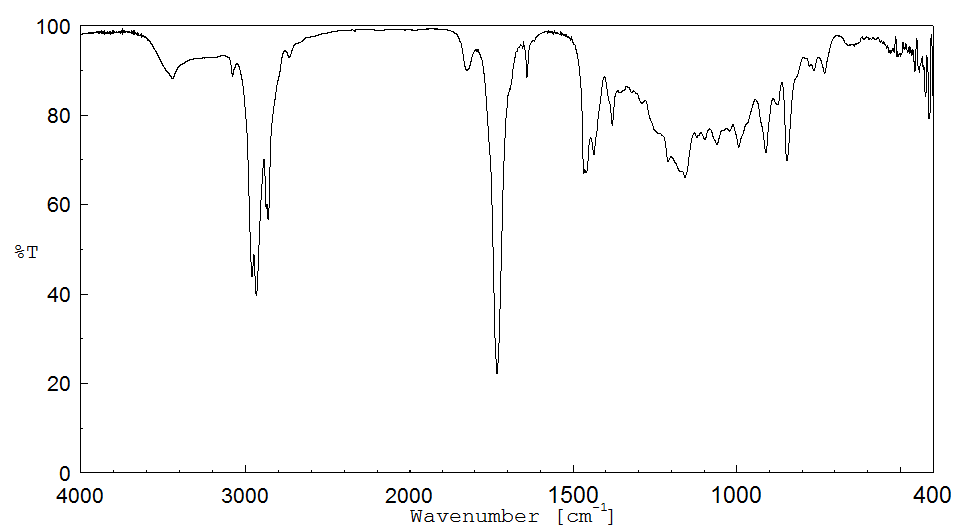
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷