苯基-α-D-吡喃葡萄糖苷 | 4630-62-0
中文名称
苯基-α-D-吡喃葡萄糖苷
中文别名
苯基-Α-D-吡喃葡萄糖苷;苯基-Alpha-D-葡萄糖苷;苯基-α-D-葡萄糖苷
英文名称
phenyl-α-D-glucopyranoside
英文别名
phenyl-alpha-D-glucopyranoside;Phenyl alpha-D-glucopyranoside;(2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-phenoxyoxane-3,4,5-triol
CAS
4630-62-0
化学式
C12H16O6
mdl
MFCD00006594
分子量
256.255
InChiKey
NEZJDVYDSZTRFS-ZIQFBCGOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172 °C
-
沸点:482.1±45.0 °C(Predicted)
-
密度:1.451±0.06 g/cm3(Predicted)
-
溶解度:甲醇(微溶)、水(微溶)
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:99.4
-
氢给体数:4
-
氢受体数:6
安全信息
-
危险品标志:C
-
安全说明:S26,S27,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2932999099
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Phenyl-O-(α-D-glucopyranosyl)-(1->4)-(α-D-glucopyranosyl)-(1->4)-D-glucopyranosid 39026-60-3 C24H36O16 580.54 (2R,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6R)-4,5-二羟基-2-(羟基甲基)-6-(苯氧基)四氢吡喃-3-基]氧基-6-(羟基甲基)四氢吡喃-3,4,5-三醇 phenyl α-maltoside 1175-37-7 C18H26O11 418.398 —— phenyl 6-O-[(ethyloxy)carbonyl]-α-D-glucopyranoside 1253394-49-8 C15H20O8 328.319 —— Phenyl 6-Deoxy-6-fluoro-α-D-glucopyranoside 87638-52-6 C12H15FO5 258.247 —— phenyl 6-O-phenoxythiocarbonyl-β-D-glucopyranoside 1395088-31-9 C19H20O7S 392.43 —— O1-phenyl-α-D-glucopyranuronic acid 17681-03-7 C12H14O7 270.239 —— phenyl 2-O-phenoxythiocarbonyl-α-D-glucopyranoside 1379776-87-0 C19H20O7S 392.43 —— phenyl-2,3,4-tri-O-benzyl-α-D-glucopyranoside 57783-69-4 C33H34O6 526.629 —— phenyl 2,3,4,6-tetra-O-benzyl-α-D-glucopyranoside 77667-56-2 C40H40O6 616.754 —— phenyl 4,6-O-benzylidene-1-O-α-D-glucopyranoside 111137-95-2 C19H20O6 344.364 —— phenyl-6-O-trityl-α-D-glucopyranoside 174194-03-7 C31H30O6 498.576 —— phenyl-2,3,4-tri-O-benzyl-6-O-trityl-α-D-glucopyranoside 762287-72-9 C52H48O6 768.95 —— phenyl 2-O-(3,5-difluorobenzensulfonyl)-α-D-glucopyranoside 1399292-80-8 C18H18F2O8S 432.399 D-葡萄糖 D-glu 2280-44-6 C6H12O6 180.158 乙基 beta-D-吡喃葡萄糖苷 (2R,3R,4S,5S,6R)-2-Ethoxy-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol 3198-49-0 C8H16O6 208.211 (2S,3r,4s,5s,6r)-2-乙氧基-6-羟基甲基四氢吡喃-3,4,5-三醇 ethyl α-D-glucopyranoside 19467-01-7 C8H16O6 208.211 —— 2,2,2-Trifluoroethyl β-D-glucopyranoside 29074-00-8 C8H13F3O6 262.183 —— 2,2,2-Trifluoroethyl α-D-glucopyranoside 29074-00-8 C8H13F3O6 262.183 - 1
- 2
反应信息
-
作为反应物:描述:苯基-α-D-吡喃葡萄糖苷 在 4,4'-二氨基二苯乙烯-2,2'-二磺酸 作用下, 以 二氯甲烷 为溶剂, 反应 120.0h, 以38%的产率得到Phenyl 4,6-Dideoxy-4,6-difluoro-α-D-galactopyranoside参考文献:名称:Fluorinated carbohydrates. 2. Selective fluorination of gluco- and mannopyranosides. Use of 2-D NMR for structural assignments摘要:DOI:10.1021/jo00172a054
-
作为产物:描述:参考文献:名称:CCCCXV。—葡糖苷。第一部分:由3:4:6-三乙酰基葡萄糖1:2-酐形成葡糖苷摘要:DOI:10.1039/jr9280003140
文献信息
-
Selective electrochemical glycosylation by reactivity tuning1作者:Robert R. France、Richard G. Compton、Benjamin G. Davis、Antony J. Fairbanks、Neil V. Rees、Jay D. WadhawanDOI:10.1039/b316728c日期:——Electrochemical glycosylation of a selenoglycoside donor proceeds efficiently in an undivided cell in acetonitrile to yield β-glycosides. Measurement of cyclic voltammograms for a selection of seleno-, thio-, and O-glycosides indicates the dependence of oxidation potential on the anomeric substituent allowing the possibility for the rapid construction of oligosaccharides by selective electrochemical activation utilising variable cell potentials in combination with reactivity tuning of the glycosyl donor. A variety of disaccharides are readily synthesised in high yield, but limitations of the use of selenoglycosides as glycosyl donors for selective glycosylation of thioglycoside acceptors are exposed. The first electrochemical trisaccharide synthesis is described.
-
Rational design of reversible inhibitors for trehalose 6-phosphate phosphatases作者:Chunliang Liu、Debra Dunaway-Mariano、Patrick S. MarianoDOI:10.1016/j.ejmech.2017.02.001日期:2017.3ortholog is 5.3 ± 0.6 μM, 70-fold smaller than the substrate Michaelis constant. The binding specificity of 9a was demonstrated using several representative sugar phosphate phosphatases from the HAD enzyme superfamily, the T6PP protein fold family of origin. Lastly, correlations drawn between T6PP active site structure, inhibitor structure and inhibitor binding affinity suggest that the aryl d-glucopyranoside在某些生物中,环境压力触发了海藻糖生物合成,海藻糖生物合成由海藻糖6-磷酸合酶和海藻糖6-磷酸磷酸酶(T6PP)共同催化。T6PP催化将海藻糖6-磷酸酯(T6P)水解为海藻糖和无机磷酸酯,是开发抗菌,抗真菌和抗蠕虫药的有希望的目标。在这里,我们报告设计,合成和评估的芳基d-吡喃葡萄糖苷6硫酸盐的库,以用作小分子T6PP抑制剂的原型。稳态动力学技术用于测量一组源自病原体马来亚布鲁氏菌(Brugia malayi),猪scar虫(Ascaris suum),多种结构上不同的T6PP直系同源物的抑制常数(K i)。结核分枝杆菌,博伊氏志贺氏菌和鼠伤寒沙门氏菌。发现这些T6PP直系同源物中最活跃的抑制剂4-正辛基苯基α - d-吡喃葡萄糖苷6-硫酸盐(9a)的结合亲和力在低微摩尔范围内。与马来西亚芽孢杆菌T6PP直系同源物的9a的K i为5.3±0.6μM,比底物Michaelis常数小70倍。9
-
Organotin‐Catalyzed Highly Regioselective Thiocarbonylation of Nonprotected Carbohydrates and Synthesis of Deoxy Carbohydrates in a Minimum Number of Steps作者:Wataru Muramatsu、Satoko Tanigawa、Yuki Takemoto、Hirofumi Yoshimatsu、Osamu OnomuraDOI:10.1002/chem.201104007日期:2012.4.16Nonprotected carbohydrates: The catalytic regioselective thiocarbonylation of carbohydrates by using organotin dichloride under mild conditions was demonstrated. The reaction afforded various deoxy saccharides in high yields and excellent regioselectivity in a minimum number of steps. The regioselectivity of the thiocarbonylation is attributed to the intrinsic character of the carbohydrates based on
-
Methods And Systems For Growing Plants Using Silicate-Based Substrates, Cultivation Of Enhanced Photosynthetic Productivity And Photosafening By Utilization Of Exogenous Glycopyranosides For Endogenous Glycopyranosyl-Protein Derivatives, And Formulations, Processes And Systems For The Same申请人:Innovation Hammer, LLC公开号:US20140331555A1公开(公告)日:2014-11-13Methods for promoting plant growth based on novel photosafening treatment regimes with glycopyranosides including glycopyranosylglycopyranosides, and aryl-a-D-glycopyranosides, and more specifically, with one or more compounds comprising terminal mannosyl-triose, optionally in the presence of light enhanced by one or more light reflecting and/or refracting members such as silicon-based substrates. Furthermore, chemical synthesis processes for the above compounds are disclosed for general application to plants. Silicate microbeads of the like are distributed over the ground or substrate in which roots of a plant are supported and planted, beneath and around a plant in a manner that light is refracted or reflected toward the phylloplane.
-
β-d-glucosidases of Sclerotium rolfsii. Substrate specificity and mode of action作者:Jai C. Sadana、Jaiprakash G. Shewale、Rajkumar V. PatilDOI:10.1016/0008-6215(83)88048-3日期:1983.7Abstract The substrate specificity and mode of action of the four pure β- d -glucosidase enzymes (EC 3.2.1.21) from Sclerotium rolfsii were studied and their contribution to cellulolysis is discussed. The enzymes are specific for substrates having the β- d configuration. The specificity of the enzymes is not restricted to the β- d -(1→4) linkage, as all four β- d -glucosidases hydrolyzed substrates having摘要研究了Sclerotium rolfsii的四种纯β-d-葡萄糖苷酶(EC 3.2.1.21)的底物特异性和作用方式,并讨论了它们对纤维素分解的贡献。所述酶对具有β-d构型的底物具有特异性。酶的特异性不限于β-d-(1→4)键,因为所有四种β-d-葡糖苷酶水解的底物均具有β-d-(1→6)-,-(1→3)和-(1→2)链接。这些酶需要严格的ad-葡萄糖构型才能发挥活性。β-d-葡糖苷酶对高度有序的纤维素(例如Avicel)没有作用,但缓慢水解的无序纤维素(磷酸溶胀的Avicel)和羧甲基纤维素,以及快速的纤维糊精,可从非还原端去除d-葡萄糖残基。纯酶表现为外切-β-d-葡聚糖葡糖水解酶。随着纤维糊精链长的增加,所有四个β-d-葡萄糖苷酶的K m值均降低。纤维素戊糖是所有四种酶的优选底物。通过用罗氏链球菌β-d-葡萄糖苷酶水解从纤维素形成d-葡萄糖的主要途径是通过较高分子量的纤维糊精进行的。
表征谱图
-
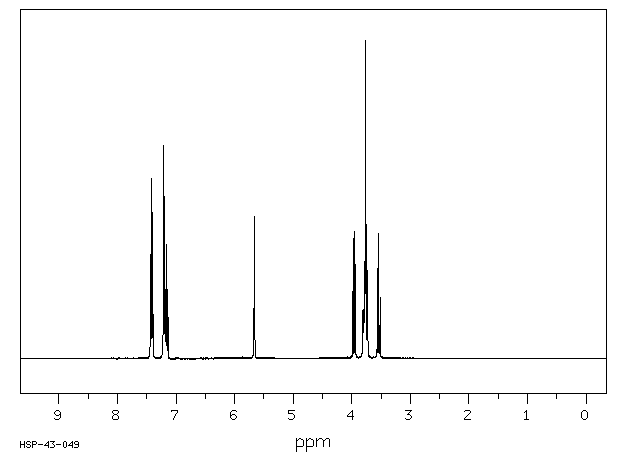
氢谱1HNMR
-
质谱MS
-
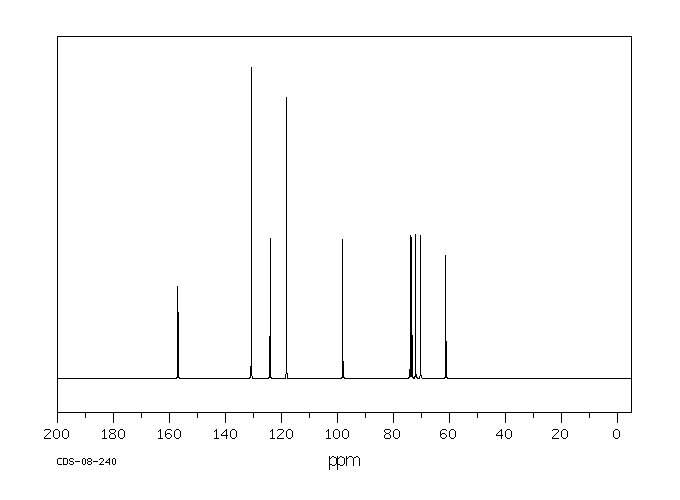
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷