(1R,S)-顺,反式菊酸-5-苄基-3-呋喃甲基-酯 | 10453-86-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56.5℃
-
沸点:bp >180° (dec)
-
密度:0.96 g/cm3
-
闪点:closed cup: 264.2°F (129°C)
-
溶解度:氯仿(微溶)、甲醇(微溶、加热)
-
物理描述:Resmethrin appears as colorless crystals or waxy solid. Insoluble in water. Used as an insecticide.
-
颜色/状态:Waxy off-white to tan solid
-
气味:Chrysanthemate odor
-
蒸汽压力:Vapor pressure, Pa at 20 °C:
-
稳定性/保质期:
遵照规格进行使用和储存,则不会发生分解。
-
分解:When heated to decomp it emits acrid and irritating fumes.
-
腐蚀性:Non-corrosive
-
折光率:Refractive index at 20 °C= 1.5287
-
保留指数:2398.5;2381.1;2393.5;2401;2413
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:25
-
可旋转键数:7
-
环数:3.0
-
sp3杂化的碳原子比例:0.41
-
拓扑面积:39.4
-
氢给体数:0
-
氢受体数:3
ADMET
安全信息
-
危险等级:9
-
危险品标志:N,Xn
-
安全说明:S60,S60/61,S61
-
危险类别码:R22,R50/53
-
WGK Germany:3
-
海关编码:29321900
-
危险品运输编号:3082
-
RTECS号:GZ1310000
-
包装等级:III
-
危险类别:6.1(b)
制备方法与用途
苄呋菊酯(resmethrin),又称FMC17370、NRDC104、NRDC119、OMS1206、OMS1800和SBPI382,商品名称包括Chrysron、Termout、灭虫菊。该杀虫剂的杀虫活性首次由M.Elliott等人报道,并先后被FMC Corp.、Penick Corp.和Sumitomo Chemical Co.,Ltd.等公司引入开发。苄呋菊酯为两个异构体的混合物,其中含有20%~30%(1RS)-顺式异构体和80%~70%(1RS)-反式异构体。工业品中两异构体总含量为84.5%。
合成方法苄呋菊酯是以丁二酸二乙酯为原料制备的,其化学合成途径如下图所示:
苄呋菊酯通过干扰神经系统的钠离子通道发挥作用。它具有强烈的触杀效果,杀虫谱广且活性高,例如对家蝇的毒力比除虫菊素高出约2.4倍,对淡色库蚊的毒力则比丙烯菊酯高约3倍。此外,其对哺乳动物的毒性低于除虫菊酯,但天然的除虫菊素有效地增效剂对其无效。
化学性质苄呋菊酯纯品为白色晶体,工业品为白色至浅黄色蜡状固体,并带有显著的除虫菊气味。其蒸汽压在200℃时为3.47×10^2 Pa,KlgP=5.43(25℃),Henry常数<8.93×10^-2 Pa·m³/mol,相对密度分别为0.958~0.968(20℃)和1.035(30℃)。水中溶解度在25℃时为37.9 μg/L;其他溶剂中其溶解度如下:丙酮30 g/100 mL,氯仿、二氯甲烷、乙酸乙酯、甲苯>50 g/100 mL,二甲苯>40 g/100 mL,乙醇、正辛醇6 g/100 mL,正己烷10 g/100 mL,异丙醚25 g/100 mL,甲醇3 g/100 mL。苄呋菊酯耐高温和氧化,但在空气和阳光下会迅速分解(比除虫菊酯慢),比旋光度[α]D为-1°~+1°,闪点为129℃。
用途苄呋菊酯具有强烈的触杀作用,杀虫效果非常高效。例如,对家蝇的毒力比除虫菊素高约2.5倍;对淡色库蚊的毒力比丙烯菊酯高出3倍左右;对德国小蠊的毒力比胺菊酯高6倍。此外,其对哺乳动物的毒性低于除虫菊素。苄呋菊酯适用于家庭、畜舍、仓库等场地的蚊、蝇、蟑螂等卫生害虫防治。
类别农药
毒性分级高毒
急性毒性- 大鼠口服LD₅₀: 1244毫克/公斤
- 小鼠口服LD₅₀: 300毫克/公斤
可燃;燃烧产生刺激烟雾
储运特性通风低温干燥;与库房食品原料分开存放
灭火剂反应信息
-
作为反应物:描述:(1R,S)-顺,反式菊酸-5-苄基-3-呋喃甲基-酯 生成 [(Z)-2-formyl-4-oxo-5-phenylpent-2-enyl] 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate参考文献:名称:RUZO, L. O.;CASIDA, J. E.;HOLDEN, I., J. AGR. AND FOOD CHEM., 1985, 33, N 4, 622-625摘要:DOI:
-
作为产物:描述:3-Furanemethanol-5-benzyl,acetate 、 菊酸甲酯 以82%的产率得到参考文献:名称:KOENIG H.; GRAF F.; WEBERNDOERFER V., LIEBIGS ANN. CHEM., 1981, NO 4, 668-682摘要:DOI:
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Thieno-pyrimidine compounds having fungicidal activity
表征谱图
-
氢谱1HNMR
-
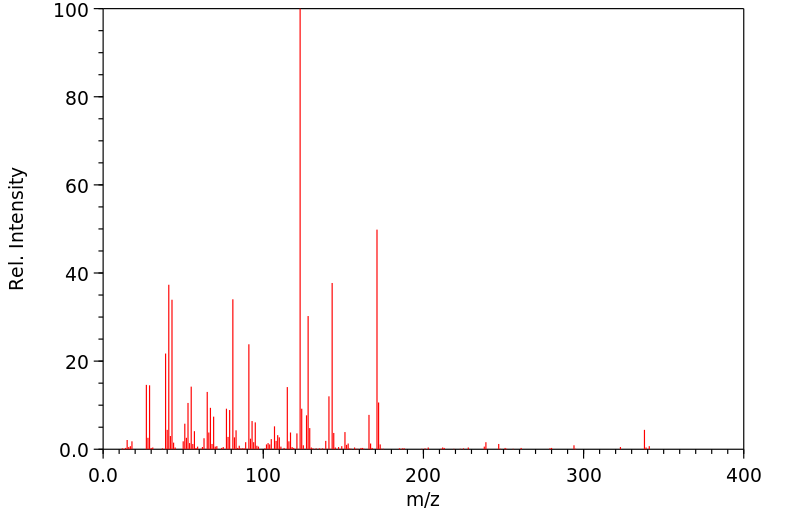
质谱MS
-
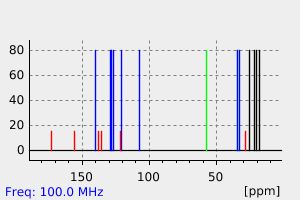
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息