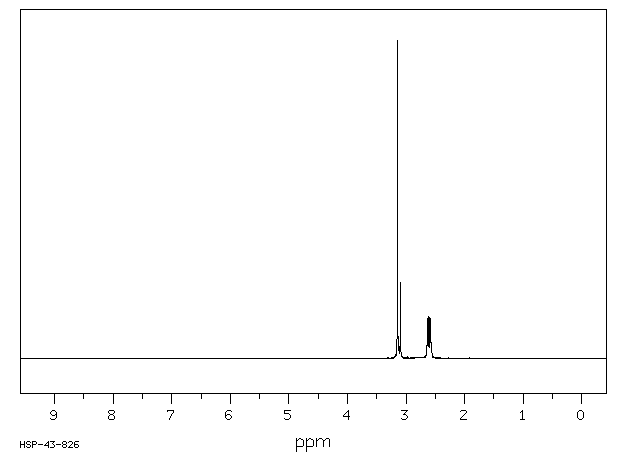
代谢
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 立即急救:确保已经进行了充分的中毒物清除。如果患者停止呼吸,开始人工呼吸,最好使用需求阀复苏器、袋阀面罩装置或口袋面罩,按训练操作。如有必要,执行心肺复苏。立即用缓慢流动的
水冲洗受污染的眼睛。不要催吐。如果发生呕吐,让患者前倾或置于左侧(如果可能的话,头部向下)以保持呼吸道畅通,防止窒息。保持患者安静,维持正常体温。寻求医疗帮助。 /毒物A和B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 基本治疗:建立专利气道(如有需要,使用口咽或鼻咽气道)。如有必要,进行吸痰。观察呼吸不足的迹象,如有需要,协助通气。通过非循环呼吸面罩以10至15升/分钟的速度给予
氧气。监测肺
水肿,如有必要,进行治疗……。监测休克,如有必要,进行治疗……。预期癫痫发作,如有必要,进行治疗……。对于眼睛污染,立即用
水冲洗眼睛。在运输过程中,用0.9%的生理盐
水(NS)持续冲洗每只眼睛……。不要使用催吐剂。对于摄入,如果患者能吞咽、有强烈的呕吐反射且不流口
水,则用
水冲洗口腔,并给予5毫升/千克,最多200毫升的
水进行稀释……。在去污后,用干燥的无菌敷料覆盖皮肤烧伤……。/毒药A和B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 高级治疗:对于无意识、严重肺
水肿或严重呼吸困难的病人,考虑进行口咽或鼻咽气管插管以控制气道。使用气囊面罩装置的正压通气技术可能有益。考虑使用药物治疗肺
水肿……。对于严重的支气管痉挛,考虑给予β受体激动剂,如
沙丁胺醇……。监测心率和必要时治疗心律失常……。开始静脉输注D5W /SRP: "保持开放",最小流量/。如果出现低血容量的迹象,使用0.9%生理盐
水(NS)或
乳酸钠林格液。对于伴有低血容量迹象的低血压,谨慎给予液体。注意液体过载的迹象……。用
地西泮或
劳拉西泮治疗癫痫……。使用
丙美卡因氢
氯化物协助眼部冲洗……。/毒物A和B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
迹象和症状...五
钠五
乙烯醇在临床测试中是非刺激性的到中等刺激性,但不是致敏剂。使用五
钠五
乙烯醇进行的人类粉刺发生(痤疮促进)测试未发现任何效果...总的来说,这些数据被认为足以支持五
钠五
乙烯醇的安全性...如在化妆品中使用。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/CA
SE REPOR
TS/ 在先前的临床研究中,报告了三名患有严重血色病且接受每日肌注Ca-
DTPA治疗以减少
铁储存的患者死亡。一名患者在接受了总共14克Ca-
DTPA后陷入昏迷并死亡,另外两名患者在每日治疗两周后死亡。这些事件与药物之间的因果关系尚未确立。 /Ca-
DTPA/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Ca-
DTPA在胃肠道吸收不良。在动物研究中,口服给药后,吸收率大约为5%。在美国的一项登记研究中,18名患者单次吸入或静脉注射1克剂量后,尿液数据显示吸入产品被吸收,并导致放射性污染物的相应排出。一项对2名通过吸入方式接受含有(14)C-
DTPA的Ca-
DTPA的人类受试者的研究显示,从肺部吸收率大约为20%。 /Ca-
DTPA/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
静脉给药后,Ca-
DTPA迅速分布到细胞外液空间。没有显著量的Ca-
DTPA渗透到红细胞或其他细胞中。没有观察到Ca-
DTPA在特定器官的积累。肾实质对
螯合剂的结合很少或没有。/Ca-
DTPA/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
给药后几小时内,Ca-
DTPA 通过肾小球滤过作用通过尿液排出体外。尚未有关于肾小管排出的记录。在检测的粪便样本中,只检测到非常少量的放射性(<3%)。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
750 kBq的(14)C-
DTPA在2个受试者中获得了血浆滞留和尿液排泄数据。放射性标记的
DTPA迅速分布到细胞外液空间,并通过肾小球过滤清除。至7小时内的血浆滞留由三个指数成分的总和表示,平均半衰期分别为1.4分钟、14.5分钟和94.4分钟。注射后24小时,血浆中的活性
水平低于检测限。在研究期间,没有检测到呼出或排泄在粪便中的活性。到24小时时,累积尿液排泄量超过注射剂量的99%。/
DTPA/
来源:Hazardous Substances Data Bank (HSDB)