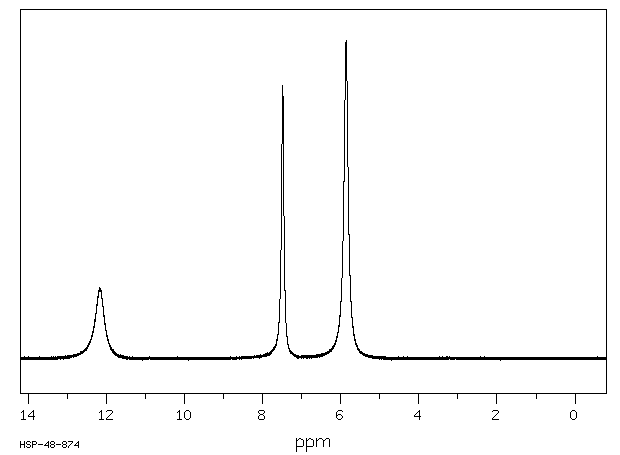
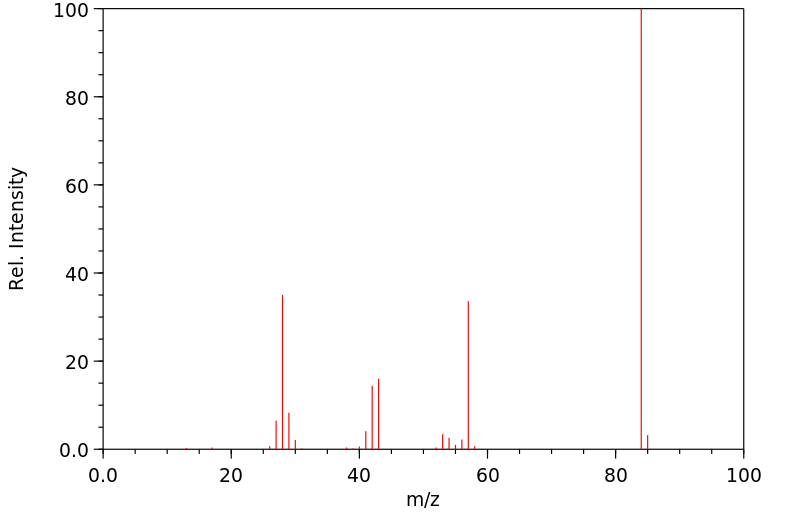
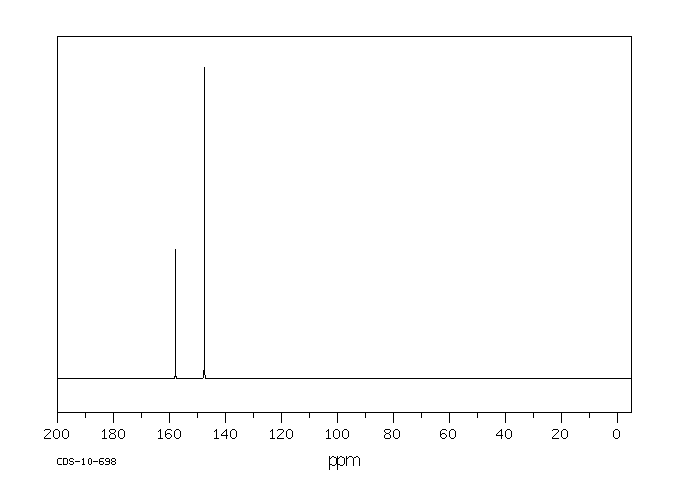
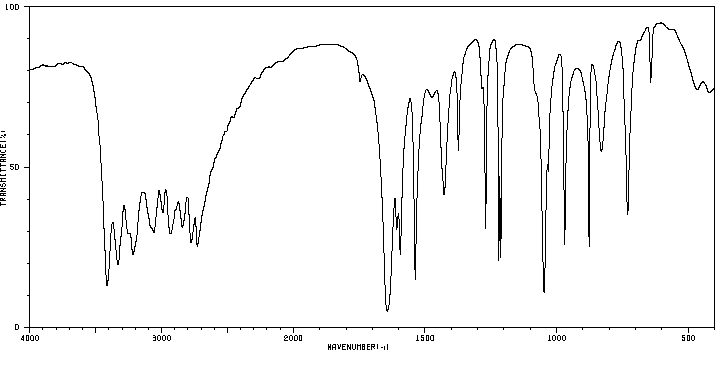
代谢
39岁女性服用20毫克/千克
氨三唑后,几小时后收集的尿液中含有没有变化的
氨三唑(100毫克/100毫升)。没有发现代谢物。
来源:Hazardous Substances Data Bank (HSDB)
代谢
植物的代谢……植物中的甘
氨酸和
丝氨酸被用于
生物合成β-(3-
氨基-
S-三唑基-1)-α-丙
氨酸。
来源:Hazardous Substances Data Bank (HSDB)
代谢
在...关于加拿大蓟的研究中,观察到了3种化合物...其中一种被鉴定为beta-(3-
氨基-
1,2,4-三唑基-1)-alpha-丙
氨酸)。
来源:Hazardous Substances Data Bank (HSDB)
代谢
由微
生物活动从
氨基三唑形成的主要代谢产物是
二氧化碳。... 大肠杆菌将3-
氨基-
1,2,4-三唑转化为代谢物,3-
氨基-
1,2,4-三唑基丙
氨酸。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
癌症分类:B2组可能的人类致癌物
来源:Hazardous Substances Data Bank (HSDB)
毒理性
美国环保局健康与环境评估办公室的人类健康评估小组对
氨基
茚进行了致癌性评估。根据他们的分析,
氨基
茚的证据权重为B2组,这一评估是基于人类证据不足和动物证据充分的结论。作为B2组
化学物质,
氨基
茚被认为是一种可能的人类致癌物。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:对于阿米特罗在人类中的致癌性,目前尚缺乏足够的证据。在实验动物中,阿米特罗的致癌性有足够的证据。总体评估:阿米特罗的致癌性对人类不可分类(第3组)。在进行评估时,工作组认为阿米特罗通过一种非
基因毒性的机制在小鼠和大鼠中产生甲状腺肿瘤,这种机制涉及干扰甲状腺
过氧化酶的功能,导致循环甲状腺激素浓度降低和甲状腺刺激激素分泌增加。因此,阿米特罗不会预期在暴露于不改变甲状腺激素内环境稳定性的浓度下,会在人类中产生甲状腺癌。工作组基于阿米特罗缺乏
基因毒性的额外考虑是,小鼠的肝肿瘤和大鼠的良性肿瘤也是通过一种非
基因毒性机制产生的。来自流行病学研究以及实验动物毒理学研究的证据提供了令人信服的证据,表明在响应甲状腺激素失衡时,啮齿动物比人类对甲状腺肿瘤的发展要敏感得多。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A3; 已确认的动物致癌物,对人类的相关性未知。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Amitrole: 有合理预期对人来说是致癌物。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
氨三唑被涂抹在兔子的皮肤上。15分钟后,它已经渗透进入血液。脂肪是...无关紧要的储存地点。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
威斯达大鼠通过胃管喂食了1毫克14C-
氨基三唑(每只大鼠)。在给药后的三天内,对呼出的空气、尿液、粪便和组织进行了放射性分析。在最初的24小时内,70至95.5%的放射性物质在尿液中找到;粪便中发现了少量可变的活动。吸收后,
氨基三唑分布到大多数身体组织中。肝和肾中发现了最大的放射性。在给药后三到四小时内,组织
水平开始下降。纸层析法揭示了在大鼠给药后不同时间取的肝切片中既有未改变的
氨基三唑也有一种未识别的代谢物。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
主要接触途径是皮肤、眼睛接触以及吸入粉末、液体和喷雾。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
气管插管的麻醉动物通过空气喷射雾化器吸入药物溶液产生的液态气溶胶。气溶胶的质量中值空气动力学直径为2.81微米,几何标准偏差为2.53。
阿米替林吸收50%所需的时间为1.3分钟。与之前报告的通过气管内注射0.1毫升药物溶液后测量的吸收速率进行比较,发现吸入气溶胶的药物吸收速度大约是气管内注射的两倍。
来源:Hazardous Substances Data Bank (HSDB)