5-溴-5'-甲酰基-2,2':5'2'-三噻吩 | 161726-69-8
中文名称
5-溴-5'-甲酰基-2,2':5'2'-三噻吩
中文别名
5''-溴-2,2':5',2''-三联噻吩-5-甲醛
英文名称
5''-bromo-[2,2':5',2''-terthiophene]-5-carbaldehyde
英文别名
5-bromo-5''-formyl-2,2':5',2''-terthiophene;5''-bromo-2,2':5',2''-terthiophene-5-carboxaldehyde;5-[5-(5-bromothiophen-2-yl)thiophen-2-yl]thiophene-2-carbaldehyde
CAS
161726-69-8
化学式
C13H7BrOS3
mdl
——
分子量
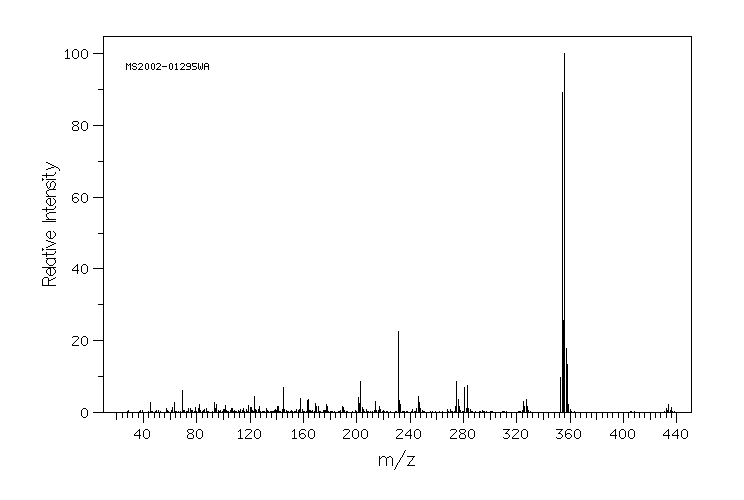
355.3
InChiKey
DSYKMLTVEGWLFR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:164 °C
-
沸点:460.0±45.0 °C(Predicted)
-
密度:1.642±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.2
-
重原子数:18
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:102
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340+P312,P305+P351+P338,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
SDS
5''-Bromo-2,2':5',2''-terthiophene-5-carboxaldehyde Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 5''-Bromo-2,2':5',2''-terthiophene-5-carboxaldehyde
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 5''-Bromo-2,2':5',2''-terthiophene-5-carboxaldehyde
Percent: >94.0%(GC)
CAS Number: 161726-69-8
Synonyms: 5-Bromo-5''-formyl-2,2':5',2''-terthiophene
Chemical Formula: C13H7BrOS3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
5''-Bromo-2,2':5',2''-terthiophene-5-
carboxaldehyde
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Very pale yellow - Yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:164 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: Soluble in : Methylene chloride
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
5''-Bromo-2,2':5',2''-terthiophene-5-
carboxaldehyde
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Sulphur oxides, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
5''-Bromo-2,2':5',2''-terthiophene-5-
carboxaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 5''-Bromo-2,2':5',2''-terthiophene-5-carboxaldehyde
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 5''-Bromo-2,2':5',2''-terthiophene-5-carboxaldehyde
Percent: >94.0%(GC)
CAS Number: 161726-69-8
Synonyms: 5-Bromo-5''-formyl-2,2':5',2''-terthiophene
Chemical Formula: C13H7BrOS3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
5''-Bromo-2,2':5',2''-terthiophene-5-
carboxaldehyde
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Very pale yellow - Yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:164 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: Soluble in : Methylene chloride
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
5''-Bromo-2,2':5',2''-terthiophene-5-
carboxaldehyde
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Sulphur oxides, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
5''-Bromo-2,2':5',2''-terthiophene-5-
carboxaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,2':5',2'-四噻吩-5-甲醛 2,2':5',2''-terthiophene-5-carboxaldehyde 7342-41-8 C13H8OS3 276.404 2-溴-5-(5-噻吩-2-基噻吩-2-基)噻吩 5-bromo-2,2':5',2''-terthiophene 94581-95-0 C12H7BrS3 327.29 5,5'-二溴-2,2'-联噻吩 5,5'-Dibromo-2,2'-bithiophene 4805-22-5 C8H4Br2S2 324.06 alpha-三联噻吩 2,2':5',2''-terthiophene 1081-34-1 C12H8S3 248.394 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-bromo-5''-hydroxymethyl-2,2':5',2''-terthiophene 162896-44-8 C13H9BrOS3 357.316 —— 5-bromo-5''-heptadecyl-2,2':5',2''-terthiophene 162896-40-4 C29H41BrS3 565.747 —— 5-formyl-5''-(1-pyrenyl)-2,2':5',2''-terthiophene 1330527-66-6 C29H16OS3 476.643 —— 5''-(4-(diphenylamino)phenyl)-2,2';5',2''-terthiophen-5-yl-carbaldehyde 1017260-46-6 C31H21NOS3 519.712 —— 5''''-(5,5-dimethyl-1,3-dioxan-2-yl)-2,2':5',2'':5'',2''':5''',2''''-quinquethiophene-5-carbaldehyde 1372699-94-9 C27H22O3S5 554.8 —— 5''-(diethylamino)-2,2':5',2''-terthiophene-5-carboxaldehyde 1043448-22-1 C17H17NOS3 347.526
反应信息
-
作为反应物:描述:5-溴-5'-甲酰基-2,2':5'2'-三噻吩 在 sodium tetrahydroborate 作用下, 以 四氢呋喃 、 甲醇 为溶剂, 反应 10.0h, 以68%的产率得到5-bromo-5''-hydroxymethyl-2,2':5',2''-terthiophene参考文献:名称:Supramolecular formation of fibrous nanostructure in donor–acceptor dyad film摘要:一种新型寡聚噻吩及其富勒烯衍生物被合成,并对其薄膜形态进行了研究。原子力显微镜(AFM)图像显示,寡聚噻吩在其液晶温度下经过热退火后形成了良好排列的纤维状纳米结构。在寡聚噻吩-富勒烯二元薄膜中,已在铸膜状态下形成了纤维。热退火进一步增强了结构的有序性,观察到一种宽度约为10纳米、长度可达200纳米的长且部分对齐的纤维状纳米结构。结合X射线分析的结果,这些特征被归因于通过寡聚噻吩基团的π-π相互作用实现的超分子自组装。同时也对这些分子的光电性能进行了研究。DOI:10.1039/b701438d
-
作为产物:描述:参考文献:名称:官能团封端的噻吩低聚物的便捷合成摘要:通过方便的三步合成,以高收率制备了具有两个,四个和六个噻吩单元的一系列α,ω-醛基封端的噻吩低聚物。紫外可见光谱数据表明,在各种溶剂中,四聚体中的π-共轭长度长于六聚体和二聚体中的π-共轭长度。DOI:10.1016/0040-4039(94)02347-e
文献信息
-
Amino(oligo)thiophene-Based Environmentally Sensitive Biomembrane Chromophores作者:Ping Yan、Aifang Xie、Meide Wei、Leslie M. LoewDOI:10.1021/jo800852h日期:2008.9.1carboxaldehyde. Side chains on these fluorophores impart a strong affinity for biological membranes. Compared with benzene analogues, these thiophene fluorophores show significant red shift in the absorption and emission spectra, offering compact red and near-infrared emitting fluorophores. More importantly, both the fluorescence quantum yields and the emission peaks are very sensitive to various environmental越来越需要使用荧光探针进行细胞成像,荧光探针发射更长的波长,以最大限度地减少生物组织的吸收、自发荧光和散射的影响。在本文中,通过 4-甲基吡啶鎓盐和各种氨基(低聚)噻吩甲醛之间的羟醛缩合,合成了一系列以氨基(低聚)噻吩供体为特征的新型环境敏感半花青染料,而后者又是从溴的胺化中获得的。 (低聚)噻吩甲醛。这些荧光团上的侧链赋予生物膜很强的亲和力。与苯类似物相比,这些噻吩荧光团在吸收和发射光谱中显示出显着的红移,提供紧凑的红色和近红外发射荧光团。更重要的是,荧光量子产率和发射峰对各种环境因素都非常敏感,例如溶剂极性或粘度、膜电位和膜组成。这些发色团还表现出很强的非线性光学特性,包括双光子荧光和二次谐波产生,它们本身对环境敏感。长波长荧光和非线性光学特性的结合使这些发色团非常适合需要在组织深处进行传感或成像的应用。它们本身对环境敏感。长波长荧光和非线性光学特性的结合使这些发色团非常适合需要在组织
-
Amino(oligo)thiophene dyes, preparation thereof, and optical methods of use申请人:The University of Connecticut公开号:US08129532B2公开(公告)日:2012-03-06Amino(oligo)thiophene dyes useful for studying the electrophysiology of organelles, cells, and tissues are described. Compared to previously known dyes, the amino(oligo)thiophene dyes exhibit improved (faster) response to membrane potential changes, as well as the ability to be excited by 1064 nanometer femtosecond pulses. Methods of preparing the amino( oligo )thiophene dyes are also described.
-
Effect of a π-linker of push–pull D–π–A donor molecules on the performance of organic photodetectors作者:Hong Chul Lim、Min-Soo Choi、Sangmin Chae、Hyo Jung Kim、Jang-Joo Kim、Jong-In HongDOI:10.1039/d0tc02168g日期:——We report organic photodetectors (OPDs) exhibiting high photocurrent density (Jph), low dark current density (Jd), and broadband external quantum efficiency (EQE) based on three push–pull type D–π–A small molecule donors (H1, H2, and H3) with different π-linker structures. Among the three donor molecules, H2 with a long π-linker had stronger intermolecular interactions and formed a polycrystalline我们报告了基于三个推挽型D–π–A小分子供体(H1)的有机光电探测器(OPD),它们显示出高光电流密度(J ph),低暗电流密度(J d)和宽带外部量子效率(EQE),H2和H3)具有不同的π-接头结构。在这三个供体分子中,具有长π接头的H2具有更强的分子间相互作用并形成多晶结构,从而在界面处产生亚空位态和陷阱位点,从而增加了J d因为从氧化铟锡电极的供体在反偏的状态易化电子注入的OPD调制的值和所述减小Ĵ的pH值。相反,由于分子间的相互作用较弱,具有短π接头的H3在薄膜中显示出非晶形态,这降低了平面异质结(PHJ)OPD和体异质结(BHJ)OPD的J d值。的BHJ OPD调制与H3在530nm处,低表现出64%的EQE宽带值Ĵ d的1.8×10值-8,和高Ĵ pH值9.6×10的值-3甲厘米-2在-3 V偏压。
-
A hydrophobic hole transporting oligothiophene for planar perovskite solar cells with improved stability作者:Lingling Zheng、Yao-Hsien Chung、Yingzhuang Ma、Lipei Zhang、Lixin Xiao、Zhijian Chen、Shufeng Wang、Bo Qu、Qihuang GongDOI:10.1039/c4cc04680c日期:——
An oligothiophene derivative with high hydrophobicity was synthesized and functioned as HTM for perovskite solar cells without an ion additive, resulting in improved device stability than that observed when using Li-TFSI doped spiro-MeOTAD.
-
An oligothiophene chromophore with a macrocyclic side chain: synthesis, morphology, charge transport, and photovoltaic performance作者:Swaminathan Venkatesan、Jianyuan Sun、Lianjie Zhang、Ashish Dubey、Andrew Sykes、Ting-Yu Lin、Yu-Chueh Hung、Qiquan Qiao、Cheng ZhangDOI:10.1039/c6ra21681a日期:——
Molecular chromophores tend to form crystals beyond nanometer sizes upon thermal aging. A novel ring-protection structure has shown promise to solve morphological stability problem of solution-processed small molecule solar cell devices.
分子色素在热老化过程中往往会形成纳米级以上的晶体。一种新颖的环保护结构已显示出解决溶液法制备的小分子太阳能电池器件形态稳定性问题的潜力。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
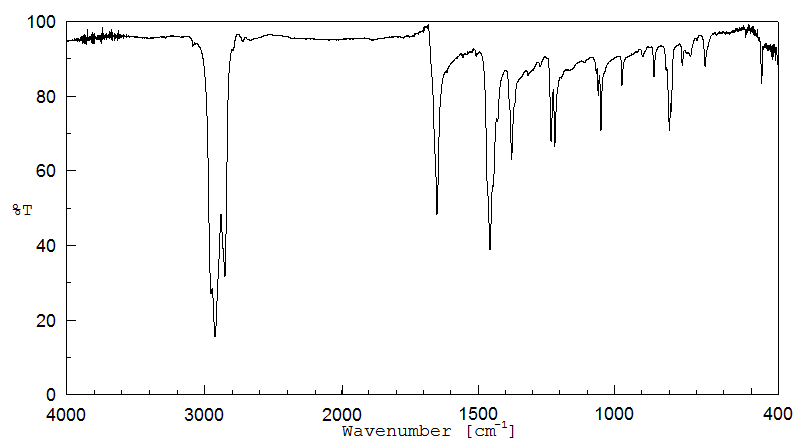
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锡烷,1,1'-(3,3'-二烷基[2,2'-二噻吩]-5,5'-二基)双[1,1,1-三甲基-
试剂5,10-Bis((5-octylthiophen-2-yl)dithieno[2,3-d:2',3'-d']benzo[1,2-b:4,5-b']dithiophene-2,7-diyl)bis(trimethylstannane)
试剂2,2'-Thieno[3,2-b]thiophene-2,5-diylbis-3-thiophenecarboxylicacid
试剂1,1'-[4,8-Bis[5-(dodecylthio)-2-thienyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并[b]噻吩,3-(2-噻嗯基)-
聚(3-己基噻吩-2,5-二基)(区域规则)
甲基[2,3'-联噻吩]-5-羧酸甲酯
牛蒡子醇 B
噻吩并[3,4-B]吡嗪,5,7-二-2-噻吩-
噻吩[3,4-B]吡嗪,5,7-双(5-溴-2-噻吩)-
十四氟-Alpha-六噻吩
三丁基(5''-己基-[2,2':5',2''-三联噻吩]-5-基)锡
α-四联噻吩
α-六噻吩
α-五联噻吩
α-七噻吩
α,ω-二己基四噻吩 5,5′-双(3-己基-2-噻吩基)-2,2′-联噻吩
α,ω-二己基六联噻吩
Α-八噻吩
alpha-三联噻吩甲醇
alpha-三联噻吩
[3,3-Bi噻吩]-2,2-二羧醛
[2,2’]-双噻吩-5,5‘-二甲醛
[2,2':5',2''-三联噻吩]-5,5''-二基双[三甲基硅烷]
[2,2'-联噻吩]-5-甲醇,5'-(1-丙炔-1-基)-
[2,2'-联噻吩]-5-甲酸甲酯
[2,2'-联噻吩]-5-乙酸,a-羟基-5'-(1-炔丙基)-(9CI)
IN1538,4,6-双(4-癸基噻吩基)-噻吩并[3,4-C][1,2,5]噻二唑(S)
C-[2,2-二硫代苯-5-基甲基]胺
6,6,12,12-四(4-己基苯基)-6,12-二氢二噻吩并[2,3-D:2',3'-D']-S-苯并二茚并[1,2-B:5,6-B']二噻吩-2,8-双三甲基锡
5’-己基-2,2’-联噻吩-5-硼酸频哪醇酯
5-辛基-1,3-二(噻吩-2-基)-4H-噻吩并[3,4-c]吡咯-4,6(5H)-二酮
5-苯基-2,2'-联噻吩
5-溴5'-辛基-2,2'-联噻吩
5-溴-5′-己基-2,2′-联噻吩
5-溴-5'-甲酰基-2,2':5'2'-三噻吩
5-溴-3,3'-二己基-2,2'-联噻吩
5-溴-3'-癸基-2,2':5',2''-三联噻吩
5-溴-2,2-双噻吩
5-溴-2,2'-联噻吩-5'-甲醛
5-氯-5'-苯基-2,2'-联噻吩
5-氯-2,2'-联噻吩
5-正辛基-2,2'-并噻吩
5-己基-5'-乙烯基-2,2'-联噻吩
5-己基-2,2-二噻吩
5-全氟己基-5'-溴-2,2'-二噻吩
5-全氟己基-2,2′-联噻吩
5-乙酰基-2,2-噻吩基
5-乙氧基-2,2'-联噻吩
5-丙酰基-2,2-二噻吩