3,5-二氟丁二酸 | 84315-23-1
中文名称
3,5-二氟丁二酸
中文别名
3,5-二氟苯乙烯酸;3,5-二氟肉桂酸
英文名称
(trans)-3-(3,5-difluorophenyl)acrylic acid
英文别名
3,5-difluorocinnamic acid;(E)-3-(3,5-difluorophenyl)acrylic acid;3,5-difluorocinammic acid;(E)-3-(3,5-difluorophenyl)prop-2-enoic acid
CAS
84315-23-1
化学式
C9H6F2O2
mdl
——
分子量
184.142
InChiKey
MBAWRXICVNIUGY-OWOJBTEDSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204-205 °C(lit.)
-
密度:1.3056 (estimate)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S26/37/39,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2916399090
-
危险性防范说明:P261,P280,P302+P352,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
| Name: | 3 5-Difluorocinnamic Acid 98% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 84315-23-1 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 84315-23-1 | 3,5-Difluorocinnamic acid | 98% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 84315-23-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: almost white to light gray
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 204 - 205 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: F2C6H3CH=CHCO2H
Molecular Weight: 184.14
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 84315-23-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,5-Difluorocinnamic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 84315-23-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 84315-23-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 84315-23-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl (E)-3-(3,5-difluorophenyl)acrylate 705250-75-5 C10H8F2O2 198.169 —— (E)-3-(3,5-difluorophenyl)acrylaldehyde 405937-99-7 C9H6F2O 168.143 3-(3,5-二氟苯基)丙醇 (E)-3-(3,5-difluorophenyl)prop-2-en-1-ol 405937-98-6 C9H8F2O 170.159 —— (E)-3-(3,5-difluorophenyl)acryloyl chloride 695187-53-2 C9H5ClF2O 202.588
反应信息
-
作为反应物:描述:3,5-二氟丁二酸 在 Petroselinum crispum phenylalanine ammonialyase (1U) ammonium hydroxide 作用下, 反应 24.0h, 以69%的产率得到L-3,5-二氟苯丙氨酸参考文献:名称:Phenylalanine Ammonia-Lyase: The Use of Its Broad Substrate Specificity for Mechanistic Investigations and Biocatalysis—Synthesis ofL-Arylalanines摘要:Several fluoro- and chlorophenylalanines were found to be good substrates of phenylalanine ammonialyase (PAL/EC 4.3.1.5) from parsley. The enantiomerically pure L-amino acids were obtained in goad yields by reaction of the corresponding cinnamic acids with 5M ammonia solution (buffered to pH 10) in the presence of PAL. The kinetic constants for nine different fluoro- and chlorophenylalanines do not provide a rigorous proof for but are consistent with the previously proposed mechanism comprising an electrophilic attack of the methylidene-imidazolone cofactor of PAL at the aromatic nucleus as a first chemical step. In the resulting Friedel-Crafts-type sigma complex the beta-protons are activated for abstraction and consequently the pro-S is abstracted by an enzymic base. Results from semiempirical calculations combined with a proposed partial active site model showed a correlation between the experimental kinetic constants and the change in polarization of the pro-S Cd-H bond and heat of formation of the ir complexes, thus making the electrophilic attack at the neutral aromatic ring plausible. Furthermore, while 5-pyrimidinylalanine was found to be a moderately good substrate of PAL, 2-pyrimidinylalanine was an inhibitor.DOI:10.1002/1521-3765(20000915)6:18<3386::aid-chem3386>3.0.co;2-5
-
作为产物:描述:参考文献:名称:Multisubstrate inhibitors of dopamine .beta.-hydroxylase. 2. Structure-activity relationships at the phenethylamine binding site摘要:1-Aralkylimidazole-2-thiones have been shown to be potent multisubstrate inhibitors of dopamine beta-hydroxylase (DBH; EC 1.14.17.1). In the present study, a series of 1-benzylimidazole-2-thiones was prepared to explore the effects of substitution in the benzyl ring on the inhibition of DBH. A detailed structure-activity relationship for in vitro activity was discovered and this was shown by a modified Hansch analysis to correlate (r = 0.91) with four key structural features of the benzyl ring: the presence of a hydroxyl at the 4-position, molar refractivity at the 3-, 4-, and 5-positions, inductive effects of the substituents at the 3-, 4-, and 5-positions, and pi-electron density. The affinity (Kis) of eight substituted inhibitors for DBH was shown to correlate (r = 0.75) with the affinity (KD) of comparably substituted tyramines for the ternary DBH-oxygen-tyramine complex. This correlate is used to support the hypothesis that binding of inhibitor to DBH occurs in a fashion that mimics the binding of tyramine substrates. The most potent inhibitors were selected for study in vivo in the spontaneously hypertensive rat model of hypertension. The changes in vascular dopamine and norepinephrine levels that resulted from oral administration of the inhibitors corresponded to the observed reduction in mean arterial blood pressure. A divergence between in vitro potency and in vivo efficacy upon oral dosing was noted and is suggested to result from an in vivo metabolic conjugation of the phenolic group of inhibitor.DOI:10.1021/jm00386a008
文献信息
-
[EN] PIPERIDINYLHYDROXYETHYLPIPERIDINES AS MODULATORS OF CHEMOKINE RECEPTORS<br/>[FR] PIPÉRIDINYLHYDROXYÉTHYLPIPÉRIDINES UTILISABLES EN TANT QUE MODULATEURS DES RÉCEPTEURS AUX CHIMIOKINES申请人:GLAXO GROUP LTD公开号:WO2009049113A1公开(公告)日:2009-04-16The present invention relates to a compound of the formula (I), or a pharmaceutically acceptable salt thereof, wherein R1-R8 and X, m, and n are defined. Compounds and compositions of the present invention are useful the treatment of atherosclerosis.本发明涉及公式(I)的化合物或其药学上可接受的盐,其中R1-R8和X、m和n已定义。本发明的化合物和组合物对动脉粥样硬化的治疗是有用的。
-
Structure-activity relationships of 8-styrylxanthines as A2-selective adenosine antagonists作者:Kenneth A. Jacobson、Carola Gallo-Rodriguez、Neli Melman、Bilha Fischer、Michel Maillard、Andrew van Bergen、Philip J. M. van Galen、Yishai KartonDOI:10.1021/jm00062a005日期:1993.57-alkylxanthines was synthesized as potential A2-selective adenosine receptor antagonists, and the potency at rat brain A1- and A2-receptors was studied in radioligand binding experiments. At the xanthine 7-position, only small hydrophobic substituents were tolerated in receptor binding. 7-Methyl analogues were roughly 1 order of magnitude more selective for A2 versus A1 receptors than the corresponding 7-H analogues合成了一系列取代的1,3,7-烷基黄嘌呤的8-苯乙烯基衍生物作为潜在的A2选择性腺苷受体拮抗剂,并在放射性配体结合实验中研究了对大鼠脑A1和A2受体的效价。在黄嘌呤7位上,受体结合中仅容忍小的疏水取代基。7-甲基类似物对A2和A1受体的选择性比相应的7-H类似物高大约1个数量级。1,3-二甲基黄嘌呤衍生物比相应的1,3-二烯丙基,二乙基或二丙基衍生物倾向于对A2-受体更具选择性。在3-(单取代)和3,5-(二取代)位置上苯环的取代是有利的。1,3,7-三甲基-8-(3-氯苯乙烯基)黄嘌呤是中等效力的(Ki vs [3H] CGS 21680为54 nM)和高度A2选择性(520倍)腺苷拮抗剂。1,3,7-三甲基-8-[(3-羧基-1-氧丙基)氨基]苯乙烯基]黄嘌呤具有很高的A2选择性(250倍),并且具有更高的水溶性(最大19 mM)。1,3-二丙基-7-甲基-8-(3,5-二甲氧基苯乙烯基)黄嘌呤是有效的(Ki
-
Semireduction of alkynoic acids via a transition metal-free α borylation-protodeborylation sequence作者:Astha Verma、R. Justin Grams、Brett P. Rastatter、Webster L. SantosDOI:10.1016/j.tet.2019.02.030日期:2019.4A method for the semi-reduction of alkynoic acids through an α-borylation and subsequent protodeborylation mechanism has been developed. The transition metal-free protocol is achieved through the activation of bis(pinacolato)diboron by an in situ generated carboxylate moiety yielding aryl acrylic acids. Our studies demonstrate an unprecedented dual role for the carboxylate anion that involves the activation
-
Molecular structure and pretilt control of photodimerized-monolayers (PDML)作者:Jawad Naciri、Devanand K. Shenoy、Kirsten Grüeneberg、Ranganathan ShashidharDOI:10.1039/b403656e日期:——We have studied the alignment of nematic liquid crystals on photo-sensitive chemisorbed monolayers. Surface modification and a single UV exposure at normal incidence resulted in photo-dimerized monolayers. A uniform, planar alignment of liquid crystals is realized on these surfaces. Chemical modification of the photo-sensitive chromophores of the monolayer allow fine-tuning of the pretilt. For a given alignment layer, there is a good correlation between the value of the pretilt and the polar properties of the liquid crystal used. Furthermore, the value of the pretilt depends on the chemical functionality at the outermost portion of the photo-alignment layer.
-
Modular Cyclopentenone Synthesis through the Catalytic Molecular Shuffling of Unsaturated Acid Chlorides and Alkynes作者:Yong Ho Lee、Elliott H. Denton、Bill MorandiDOI:10.1021/jacs.0c10832日期:2020.12.16general strategy for the intermolecular synthesis of polysubstituted cyclopentenones using palladium catalysis. Overall, this reaction is achieved via a molecular shuffling process involving an alkyne, an α,β-unsaturated acid chloride, which serves as both the alkene and carbon monoxide source, and a hydrosilane to create three new C-C bonds. This new carbon monoxide-free pathway delivers the products
表征谱图
-
氢谱1HNMR
-
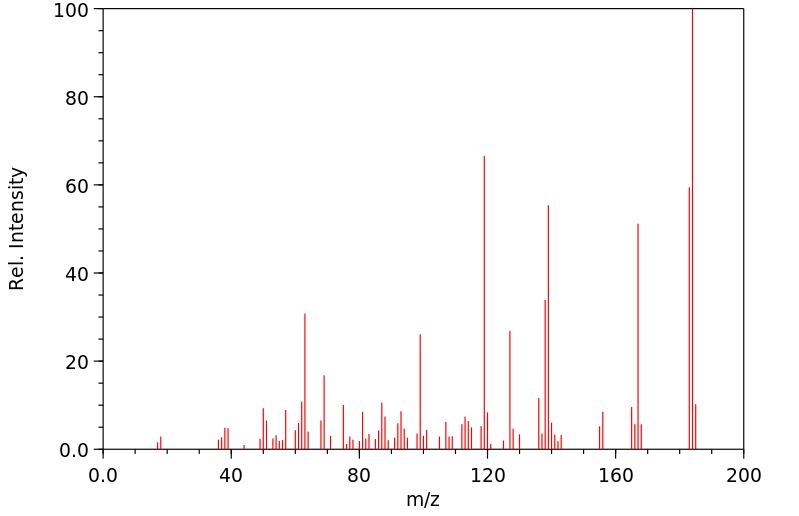
质谱MS
-
碳谱13CNMR
-
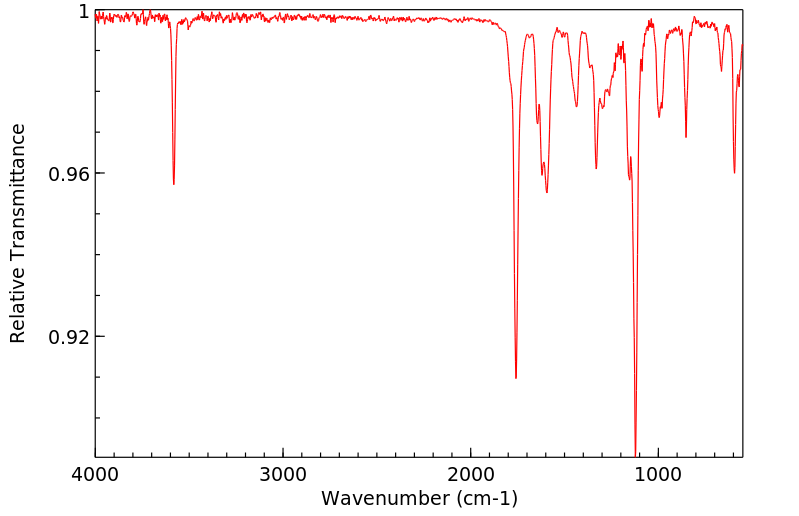
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30