代谢
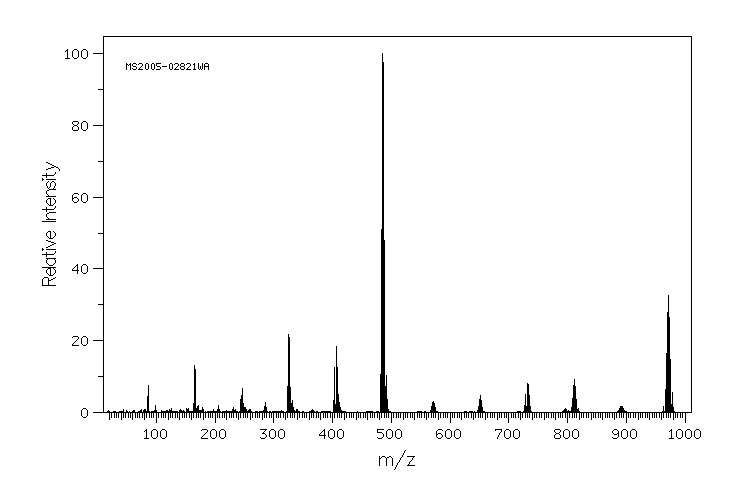
至少在暴露于DBDPE的大鼠中观察到了七种未知化合物,这表明DBDPE在大鼠体内发生了生物转化。通过比较气相色谱/电子电离质谱(GC/EI-MS)和气相色谱/电子捕获负离子化质谱(GC/ECNI-MS)分析中的DBDPE光降解实验得到的脱溴产物的相对保留时间和全扫描质谱,鉴定了这些化合物。结果显示,在大鼠体内观察到的主要代谢途径并不是DBDPE向低溴代BDPEs的脱溴反应。通过GC/EI-MS,两种代谢物被暂时鉴定为MeSO(2)-nona-BDPE和EtSO(2)-nona-BDPE,但它们的结构需要通过其他技术和标准样品进一步确认。此外,大鼠对DBDPE和BDE-209及其代谢物的生物反应证据是不同的。
At least seven unknown compounds were observed in the DBDPE-exposed rats, indicating that DBDPE biotransformation occurred in rats. These compounds were identified by comparing relative retention times and full-scan mass spectra of DBDPE debrominated products from a photolytic degradation experiment using GC/EI-MS and GC/ECNI-MS analysis. The results showed that debromination of DBDPE to lower brominated BDPEs were not the primary metabolic pathway observed in rats. Two of the metabolites were proposed tentatively as MeSO(2)-nona-BDPE and EtSO(2)-nona-BDPE using GC/EI-MS, but their structures require further confirmation by other techniques and authentic standards. In addition, evidence of a biological response to DBDPE and BDE-209 and their metabolites in rats are different.
来源:Hazardous Substances Data Bank (HSDB)