宾雪德拉氏绿隐色碱 | 637-31-0
中文名称
宾雪德拉氏绿隐色碱
中文别名
双(4-二甲基氨基苯基)胺;N,N,N',N'-四甲基二氨基二苯胺;4,4-二(二甲氨基)二苯胺;双(4-二甲氨基苯基)胺
英文名称
4,4'-bis(dimethylamino)diphenylamine
英文别名
bis(4-dimethylaminophenyl)amine;bis(p-dimethylaminophenyl)amine;Bindschedler's green leuco base;1-N-[4-(dimethylamino)phenyl]-4-N,4-N-dimethylbenzene-1,4-diamine
CAS
637-31-0
化学式
C16H21N3
mdl
MFCD00059291
分子量
255.363
InChiKey
ZPGZNKGKCSYBGK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:121°C
-
沸点:416.7±30.0 °C(Predicted)
-
密度:1.103±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:18.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2921590090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
Bindschedler's Green Leuco Base Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Bindschedler's Green Leuco Base
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Bindschedler's Green Leuco Base
Percent: >98.0%(LC)(T)
CAS Number: 637-31-0
Synonyms: Bis(4-dimethylaminophenyl)amine , Leuco Bindschedler's Green , N,N,N',N'-
Tetramethyldiaminodiphenylamine
Chemical Formula: C16H21N3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Bindschedler's Green Leuco Base
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: Pale gray - Reddish gray
Odor: No data available
pH: No data available
Melting point/freezing point:121 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Conditions to avoid: Air-sensitive
Bindschedler's Green Leuco Base
Section 10. STABILITY AND REACTIVITY
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
No data available
log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
Bindschedler's Green Leuco Base
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Bindschedler's Green Leuco Base
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Bindschedler's Green Leuco Base
Percent: >98.0%(LC)(T)
CAS Number: 637-31-0
Synonyms: Bis(4-dimethylaminophenyl)amine , Leuco Bindschedler's Green , N,N,N',N'-
Tetramethyldiaminodiphenylamine
Chemical Formula: C16H21N3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Bindschedler's Green Leuco Base
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: Pale gray - Reddish gray
Odor: No data available
pH: No data available
Melting point/freezing point:121 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Conditions to avoid: Air-sensitive
Bindschedler's Green Leuco Base
Section 10. STABILITY AND REACTIVITY
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
No data available
log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
Bindschedler's Green Leuco Base
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基-对苯二胺 4-amino-N,N-dimethylaniline 99-98-9 C8H12N2 136.197 N1-[4-(二甲基氨基)苯基]-N4,N4-二甲基-N1-亚硝基-1,4-苯二胺 bis-(4-dimethylamino-phenyl)-nitroso-amine 13916-80-8 C16H20N4O 284.361 N,N-二甲基苯胺 N,N-dimethyl-aniline 86362-18-7 C8H11N 121.182 —— tetrakis-(4-dimethylamino-phenyl)-hydrazine 1990-18-7 C32H40N6 508.71 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4,4'-bis(dimethylamino)triphenylamine 55087-74-6 C22H25N3 331.461 —— bis-(4-dimethylamino-phenyl)-methyl-amine 4979-36-6 C17H23N3 269.39 —— 4-N-[4-(dimethylamino)phenyl]-4-N-(4-fluorophenyl)-1-N,1-N-dimethylbenzene-1,4-diamine 64634-56-6 C22H24FN3 349.451 N1-[4-(二甲基氨基)苯基]-N4,N4-二甲基-N1-亚硝基-1,4-苯二胺 bis-(4-dimethylamino-phenyl)-nitroso-amine 13916-80-8 C16H20N4O 284.361 —— tetrakis-(4-dimethylamino-phenyl)-hydrazine 1990-18-7 C32H40N6 508.71
反应信息
-
作为反应物:描述:宾雪德拉氏绿隐色碱 在 吡啶 、 乙醚 、 nitrogen oxides 、 silver(l) oxide 作用下, 生成 N1-[4-(二甲基氨基)苯基]-N4,N4-二甲基-N1-亚硝基-1,4-苯二胺参考文献:名称:Wieland, Chemische Berichte, 1915, vol. 48, p. 1110摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 tin(ll) chloride 作用下, 生成 宾雪德拉氏绿隐色碱参考文献:名称:Bindschedler, Chemische Berichte, 1883, vol. 16, p. 871摘要:DOI:
文献信息
-
Use of Rylene Derivatives as Photosensitizers in Solar Cells申请人:Pschirer Neil Gregory公开号:US20080269482A1公开(公告)日:2008-10-30Use of rylene derivatives I with the following definition of the variables: X together both —COOM; Y a radical -L-NR 1 R 2 (y1) -L-Z-R 3 (y2) the other radical hydrogen; together both hydrogen; R is optionally substituted (het)aryloxy, (het)arylthio; P is —NR 1 R 2 ; B is alkylene; optionally substituted phenylene; combinations thereof; A is —COOM; —SO 3 M; —PO 3 M 2 ; D is optionally substituted phenylene, naphthylene, pyridylene; M is hydrogen; alkali metal cation; [NR 5 ] 4 + ; L is a chemical bond; optionally indirectly bonded, optionally substituted (het)arylene radical; R 1 , R 2 are optionally substituted (cyclo)alkyl, (het)aryl; together optionally substituted ring comprising the nitrogen atom; Z is —O—; —S—; R 3 is optionally substituted alkyl, (het)aryl; R′ is hydrogen; optionally substituted (cyclo)alkyl, (het)aryl; R 5 is hydrogen; optionally substituted alkyl (het)aryl; m is 0, 1, 2; n, p m=0: 0, 2, 4 where: n+p=2, 4, if appropriate 0; m=1: 0, 2, 4 where: n+p=0, 2, 4; m=2: 0, 4, 6 where: n+p=0, 4, 6, or of mixtures thereof as photosensitizers in solar cells.使用以下变量定义的莱伦衍生物I的用途: X一起 两者都是-COOM; Y是一个基团 -L-NR 1 R 2 (y1) -L-Z-R 3 (y2) 另一个基团是氢; 一起 两者都是氢; R可选择地被取代为(het)芳氧基,(het)芳基硫基; P是-NR 1 R 2 ; B是烷基; 可选择地被取代的苯基; 它们的组合; A是-COOM; -SO 3 M; -PO 3 M 2 ; D可选择地被取代为苯基,萘基,吡啶基; M是氢; 碱金属阳离子; [NR 5 ] 4 + ; L是化学键; 可选择地间接键合,可选择地被取代的(het)芳基基团; R 1 ,R 2 可选择地被取代的(环)烷基,(het)芳基; 一起可选择地被取代的环,其中包括氮原子; Z是-O-; -S-; R 3 可选择地被取代的烷基,(het)芳基; R′是氢; 可选择地被取代的(环)烷基,(het)芳基; R 5 是氢; 可选择地被取代的烷基(het)芳基; m为0, 1, 2; n, p, m=0: 0, 2, 4其中:n+p=2, 4,如果适用为0; m=1: 0, 2, 4其中:n+p=0, 2, 4; m=2: 0, 4, 6其中:n+p=0, 4, 6, 或者作为太阳能电池中的光敏剂的混合物。
-
[EN] SUBSTITUTED DIARYLAMINES AND USE OF SAME AS ANTIOXIDANTS<br/>[FR] DIARYLAMINES SUBSTITUÉES ET UTILISATION DE CELLES-CI EN TANT QU'ANTIOXYDANTS申请人:UNIV KINGSTON公开号:WO2012162818A1公开(公告)日:2012-12-06The present invention relates to substituted heteroaromatic dianlamine compounds of Formula I and II, their pharmaceutically acceptable salts, and compositions thereof useful as antioxidants, wherein each of X, Y and Z are independently a carbon or nitrogen atom; R1 and R2 are each independently a hydrogen or an electron donating group, but are not both hydrogen, and wherein R1 and R2 are each bonded to a carbon atom in their own respective aryl ring.本发明涉及Formula I和II的替代杂环二胺化合物,它们的药用盐以及作为抗氧化剂有用的组合物,其中X、Y和Z中的每一个都是独立的碳或氮原子;R1和R2分别是氢或电子给体基团,但两者不都是氢,并且R1和R2分别与它们自己各自的芳香环中的碳原子结合。
-
Condensations of 1,4-Cyclohexanediones and Secondary Aromatic Amines. II.<i>N</i>-Phenylation of Diarylamines作者:Kazuo Haga、Katsumasa Iwaya、Ryohei KanekoDOI:10.1246/bcsj.59.803日期:1986.3The condensations of 1,4-cyclohexanedione with several diphenylamines were investigated in order to determine the limit of the utility of this reaction for the N-phenylation of aromatic secondary amines. 4-Methoxy-, 4,4′-dimethyl-, and 4,4′-dibromodiphenylamines produced their N-phenylated compounds in fairly good yields, but 4-hydroxy-, 3-methoxy-, and 4,4′-bis(dimethylamino)diphenylamines produced
-
Nucleophilic Aromatic Substitution Reactions of<i>meso</i>-Bromosubporphyrin: Synthesis of a Thiopyrane-Fused Subporphyrin作者:Daiki Shimizu、Hirotaka Mori、Masaaki Kitano、Won-Young Cha、Juwon Oh、Takayuki Tanaka、Dongho Kim、Atsuhiro OsukaDOI:10.1002/chem.201405110日期:2014.12.1meso‐Bromosubporphyrin undergoes nucleophilic aromatic substitution (SNAr) reactions with arylamines, diarylamines, phenols, ethanol, thiophenols, and n‐butanethiol in the presence of suitable bases to provide the corresponding substitution products. The SNAr reactions also proceed well with pyrrole, indole, and carbazole to provide substitution products in moderate to good yields. Finally, the SNAr
-
Substituted furopyridinones and furopyrazinones申请人:——公开号:US04211872A1公开(公告)日:1980-07-08Substituted furopyridinones and furopyrazinones which are useful as color formers in pressure-sensitive carbonless duplicating systems and thermal marking systems are prepared by reacting diphenylamines with substituted benzoyl (or 3-indolylcarbonyl)pyridinecarboxylic acids and substituted benzoyl (or 3-indolylcarbonyl)pyrazinecarboxylic acids, respectively.
表征谱图
-
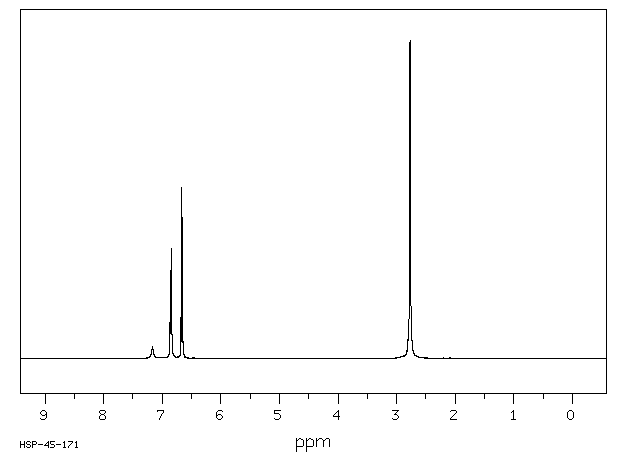
氢谱1HNMR
-
质谱MS
-
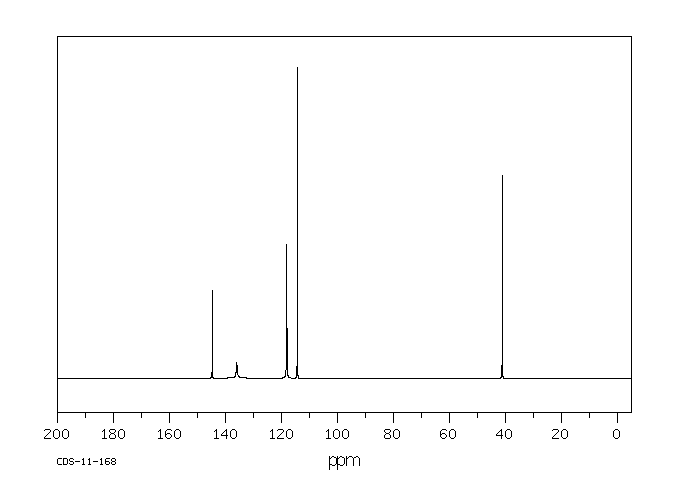
碳谱13CNMR
-
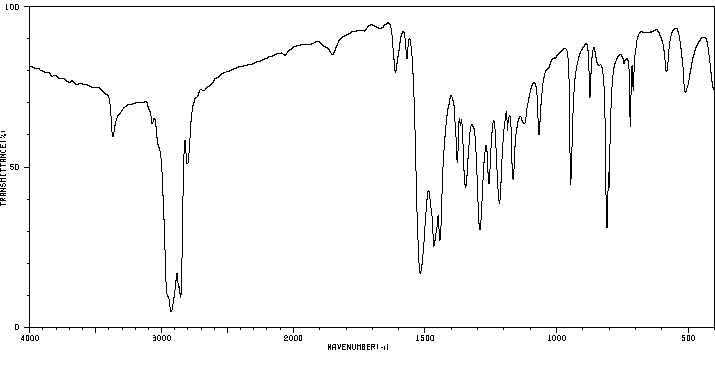
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷