亚乙基环庚烷 | 10494-87-8
中文名称
亚乙基环庚烷
中文别名
——
英文名称
Aethyliden-cycloheptan
英文别名
Ethylidencycloheptan;Ethylidenecycloheptane
CAS
10494-87-8
化学式
C9H16
mdl
MFCD00046509
分子量
124.226
InChiKey
MLFGNFYXTYMGAT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:79-81 °C(Press: 10 Torr)
-
密度:0.878±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.777
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:描述:亚乙基环庚烷 在 cobalt(II) bis[bis((trifluoromethyl)sulfonyl)amide] 、 氢气 、 zinc trifluoromethanesulfonate 、 碳酸氢钠 、 间氯过氧苯甲酸 、 1,1,1-三(二苯基膦甲基)乙烷 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 80.0 ℃ 、4.0 MPa 条件下, 反应 17.33h, 生成 1-cycloheptylethanol参考文献:名称:通过钴的环氧催化加氢,一般区域选择性合成醇。摘要:提出了一种简单的合成抗马尔可夫尼可夫醇的方法。通过在Zn(OTf)2作为添加剂的情况下使用特定的钴三光配合物,可以高收率和选择性地进行环氧化物的氢化。所描述的方案显示了广泛的底物范围,包括多取代的内部和末端环氧化物,以及良好的官能团耐受性。各种天然产物衍生物,包括类固醇,萜类和倍半萜类,都以中等至优异的产率获得了相应的醇。DOI:10.1002/anie.202002844
-
作为产物:参考文献:名称:Borsdorf,R.; Olesch,B., Journal fur praktische Chemie (Leipzig 1954), 1967, vol. 36, p. 165 - 169摘要:DOI:
文献信息
-
Asymmetric Synthesis of Dihydropyranones via Gold(I)‐Catalyzed Intermolecular [4+2] Annulation of Propiolates and Alkenes作者:Hanbyul Kim、Su Yeon Choi、Seunghoon ShinDOI:10.1002/anie.201807514日期:2018.10Intermolecular asymmetric gold catalysis involving alkyne activation presents a significant challenge due to its distinct mechanistic mode from other metals. Herein, we report a highly enantioselective synthesis of α,β‐unsaturated δ‐lactones from [4+2] annulation of propiolates and alkenes in upto 95 % ee. Notably, for the desired chiral recognition, the choice of 1,1,2,2‐tetrachloroethane as solvent
-
Asymmetric catalysis of carbonyl-ene and aldol reactions with fluoral by chiral binaphthol-derived titanium complex作者:Koichi Mikami、Tomoko Yajima、Tsuyoshi Takasaki、Satoru Matsukawa、Masahiro Terada、Tadafumi Uchimaru、Masamichi MarutaDOI:10.1016/0040-4020(95)00862-3日期:1996.1The chiral titanium complex-catalyzed ene-type reaction and the Mukaiyama aldol reaction with fluoral are shown to serve as an efficient route to the enantioselective and diastercoselective synthesis of CF3-substituted components of biological and synthetic importance.
-
Reaction of p-nitrobenzenesulfonyl azide with alkylidenecycloalkanes作者:Samuel P. McManus、Margarita Ortiz、Rudolph A. AbramovitchDOI:10.1021/jo00315a023日期:1981.1
-
Diastereoselective and enantioselective glyoxylate-ene reaction catalyzed by new class of binaphthol-derived titanium complex作者:Masahiro Terada、Yukihiro Motoyama、Koichi MikamiDOI:10.1016/s0040-4039(00)73470-8日期:1994.9Diastereo- and enantioselective carbony-ene reaction of glyoxylate (2) with trisubstituted olefins (3) catalyzed by chiral titanium complexes (1a), derived from 6,6'-dibromo-1,1'-bi-2-naphthol and disopropoxytitanium dihalides, is found to provide syn-diastereomers exclusively along with a high level of enantioselectivity.
-
<b>Proximity Effects. XXXIII. Solvolysis of <b><i>trans</i></b>-Bicyclo[6.1.0]nonane</b>作者:Arthur C. Cope、Jeffrey K. HechtDOI:10.1021/ja00895a019日期:1963.6
表征谱图
-
氢谱1HNMR
-
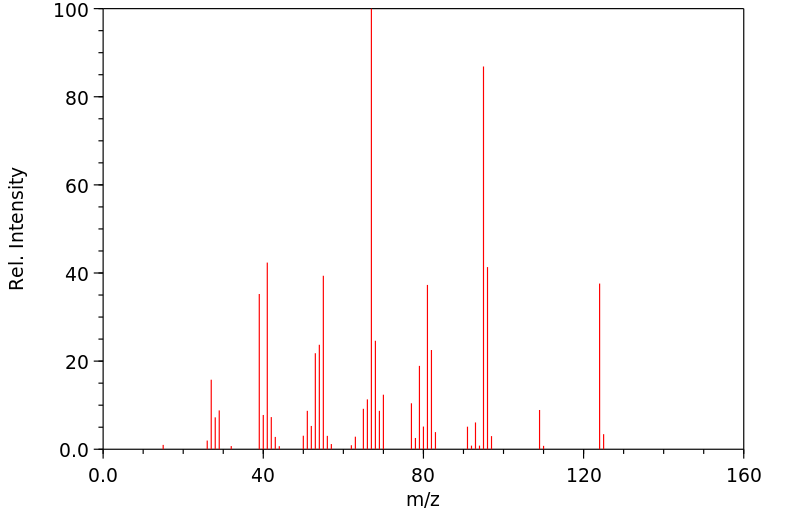
质谱MS
-
碳谱13CNMR
-
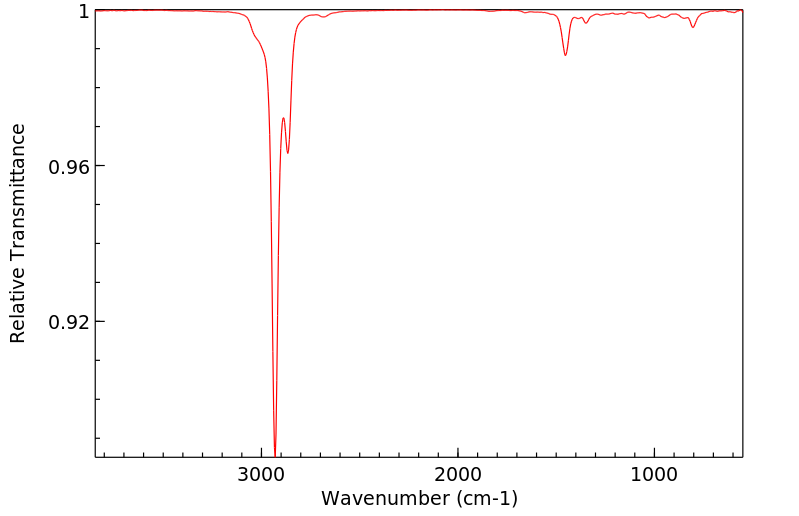
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-