(2E)-3-[4-(2-羟基乙氧基)苯基]丙烯酸 | 60345-99-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:66.8
-
氢给体数:2
-
氢受体数:4
安全信息
-
海关编码:2918990090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-香豆酸 p-Coumaric Acid 501-98-4 C9H8O3 164.161 对羟基肉桂酸 p-Coumaric acid 7400-08-0 C9H8O3 164.161 4-(2-羟基乙氧基)苯甲醛 4-(2-hydroxy-ethoxy)-benzaldehyde 22042-73-5 C9H10O3 166.177
反应信息
-
作为反应物:描述:参考文献:名称:The localization of type 2 diabetes susceptibility gene loci in northern Chinese Han families摘要:We conducted a genome-wide scan, in which 358 well distributed fluorescent dye-labeled microsatellite marker sets were applied in 32 Chinese Han type 2 diabetes families from Northern China to search for the susceptibility gene loci. The data collected from screening all the chromosomes of genome were genotyped by using genescan and genotyping software, then, parametric and non-parametric multipoint test, and affected sib-pair analysis as well, were used to analyze the data. We identified some susceptibility gene loci residing in chromosomes 1,12,18,20, respectively, or precisely, located around D1S214, D1S207, D1S218, D1S235, D12S336, D18S61 and D20S118. The comparison of this result with those from other regions and races reflected the complexity and heterogeneity of type 2 diabetes.DOI:10.1007/bf02886269
-
作为产物:参考文献:名称:内酯和生物芳烃通过同时开环聚合/缩聚的共聚摘要:内酯和生物芳族羟基酸的同时开环聚合/缩聚反应产生具有改善和受控的热性能的无规共聚物。DOI:10.1039/c6gc03238a
文献信息
-
Imidazo [1,2-a] pyridines and their pharmaceutical use申请人:Fujisawa Pharmaceutical Co., Ltd公开号:US05574042A1公开(公告)日:1996-11-12The invention relates to novel bradykinin antagonists of the formula: ##STR1## wherein R.sup.1 is halogen, R.sup.2 and R.sup.3 are each hydrogen, lower alkyl, halo(lower)alkyl or acyl, R.sup.4 is aryl having suitable substituent(s), or a heterocyclic group optionally having suitable substituent(s), Q is O or N--R.sup.11, in which R.sup.11 is hydrogen or acyl, and A is lower alkylene, and pharmaceutically acceptable salts thereof.这项发明涉及公式为:##STR1##的新型激肽酶抑制剂,其中R.sup.1是卤素,R.sup.2和R.sup.3分别是氢、较低烷基、卤代(较低)烷基或酰基,R.sup.4是具有适当取代基的芳基,或者是具有适当取代基的杂环基,Q是O或N--R.sup.11,其中R.sup.11是氢或酰基,A是较低烷基,以及其药学上可接受的盐。
-
Light-sensitive, unsaturated polymeric maleic and acrylic derivatives申请人:EASTMAN KODAK CO公开号:US02824084A1公开(公告)日:1958-02-18
4 - (g - Hydroxypropoxy)benzaldehyde is prepared by reacting p-hydroxybenzaldehyde with 3-bromopropanol. 4 - Hydroxyethoxybenzal - 41 - bromoacetophenone is prepared by reacting 4-(b -hydroxy-ethoxy)benzaldehyde with p-bromoacetophenone. 4 - Hydroxyethoxybenzalanisalacetone is prepared by reacting 4-(b -hydroxyethoxy)benzaldehyde with anisalacetone. 4-Hydroxyethoxycinnamic acid is prepared by reacting 4-(b -hydroxyethoxy)benzaldehyde with malonic acid. Ethyl 4-hydroxyethoxycinnamate is prepared by reacting 4-(b -hydroxyethoxy)benzaldehyde with ethyl hydrogen malonate.ALSO:Linear polymers comprise 20 to 100% by weight of recurring units of the form <;FORM:0825948/IV (a)/1>; or <;FORM:0825948/IV (a)/2>; wherein the benzene nucleus may contain alkyl or alkoxy substituents, R represents a styrene, vinyl ester, isopropenyl ester, alkyl acrylate, alkyl methacrylate, vinyl alkyl ether or ethylene unit, D represents either -OR1O-or -NH- where R1 is an alkylene group containing 1 to 4 carbon atoms, R2 represents a hydrogen atom, alkyl, nitro, cyano or -COOR4 group and A represents -OH, -R4, -OR4, -COOR4, -CH=CH-C6H4OR4, -C6H4-C6H5, -(CH=CH)n-C6H5 where n is 1 or 2, or <;FORM:0825948/IV (a)/3>; where m is 1 or 2 and X represents a hydrogen or halogen atom, -NO2, -CN, -R4, -OR4, -COOH, -COOR4, -CONH2 or <;FORM:0825948/IV (a)/4>; wherein R4 represents an alkyl group, the alkyl groups containing 1 to 4 carbon atoms. The polymers may be prepared by reacting maleic anhydride interpolymers or polymethacrylic anhydride with hydroxyalkoxybenzaldehydes or aminobenzaldehydes, and thereafter reacting with compounds containing active methyl or methylene groups capable of reacting with aldehyde groups. Alternatively the aldehydes may be reacted with the compounds containing active methyl or methylene groups and the products reacted with the maleic anhydride or methacrylic anhydride polymers. The reaction with the polymer may be effected in a liquid nitrogen base, e.g. pyridine. Lists of aromatic aldehydes and compounds reactable therewith are given and examples describe the reaction of of styrene-maleic anhydride interpolymers, with (2) 4 - (b - hydroxyethoxy) benzaldehyde and acetophenone or with 4-(b -hydroxyethoxy) benzalacetophenone, (3) 4-(lambda-hydroxypropoxy)-benzaldehyde and acetophenone or with 4-(g -hydroxypropoxy) benzalacetophenone, (6) 4-hydroxyethyoxybenzal - 41 - bromo - acetophenone, (8) 4-hydroxyethoxybenzalanisalacetone, (12) 4-hydroxyethoxycinnamic acid, (14) ethyl 4 - hydroxyethoxycinnamate, (16) 4-hydroxyethoxybenzal - 31 - nitroacetophenone, (18) 4 - hydroxyethoxy - a - cyanocinnamic acid, (19) 4 - hydroxyethoxybenzal - 41 -phenylacetophenone, and (22) 3-aminobenzalacetophenone; polymethacrylic anhydride with (4) 4 - (lambda - hydroxypropoxy) benzaldehyde and acetophenone, (10) 4-hydroxyethoxybenzalanisalacetone, and (21) 4-(b -hydroxyethoxy) benzalpyruvic acid; and isopropenyl acetatemaleic anhydride interpolymers, with (9) 4-hydroxyethoxybenzalanisalacetone. The products may be dissolved in solvents, e.g. acetone, dioxane, methyl ethyl ketone, pyridine, the monomethyl and monoethyl ethers of glycol and esters thereof, and chlorinated hydrocarbons, and coated on supports, e.g. of aluminium, zinc, copper, magnesium, paper, surface hydrolysed cellulose acetate or casein, and used in photographic and photomechanical reproduction. They may be sensitized, e.g. with 2-benzoylmethylene - 1 - methyl - b - naphthothiazoline, and, after exposure, images developed with a solvent, e.g. dilute ammonium hydroxide solution, aqueous acetone or methyl ethyl ketone.
4-(g-羟丙氧基)苯甲醛是通过将对羟基苯甲醛与3-溴丙醇反应制备而成。4-羟乙氧基苯甲醛-41-溴乙酰苯是通过将4-(b-羟乙氧基)苯甲醛与对溴乙酰苯反应制备而成。4-羟乙氧基苯乙酮缩酮是通过将4-(b-羟乙氧基)苯甲醛与缩酮反应制备而成。4-羟乙氧基肉桂酸是通过将4-(b-羟乙氧基)苯甲醛与丙二酸反应制备而成。4-羟乙氧基肉桂酸乙酯是通过将4-(b-羟乙氧基)苯甲醛与乙基氢丙二酸酯反应制备而成。另外:线性聚合物包含20-100%的重复单元,其形式为<;FORM:0825948/IV(a)/1>或<;FORM:0825948/IV(a)/2>,其中苯环核可以含有烷基或烷氧基取代基,R代表苯乙烯、乙烯酸酯、异丙烯酸酯、烷基丙烯酸酯、烷基甲基丙烯酸酯、乙烯基烷氧基或乙烯基单元,D代表-OR1O-或-NH-,其中R1是含有1-4个碳原子的烷基,R2代表氢原子、烷基、硝基、氰基或-COOR4基,A代表-OH、-R4、-OR4、-COOR4、-CH=CH- OR4、-C6H4-C6H5、-(CH=CH)n- ,其中n为1或2,或<;FORM:0825948/IV(a)/3>,其中m为1或2,X代表氢原子或卤素原子、-NO2、-CN、-R4、-OR4、-COOH、-COOR4、-CONH2或<;FORM:0825948/IV(a)/4>,其中R4代表烷基,烷基含有1-4个碳原子。聚合物可以通过将马来酸酐互缔物或聚甲基丙烯酸酐与羟基烷氧基苯甲醛或氨基苯甲醛反应制备而成,然后与含有活性甲基或亚甲基基团的化合物反应,这些化合物能够与醛基反应。或者将醛类与含有活性甲基或亚甲基基团的化合物反应,然后与马来酸酐或甲基丙烯酸酐聚合物反应。与聚合物的反应可以在液态氮基中进行,例如吡啶。给出了芳香醛和可反应化合物的列表,例如描述了苯乙烯-马来酸酐互缔物的反应,其中与(2)4-(b-羟乙氧基)苯甲醛和乙酰苯或4-(b-羟乙氧基)苯甲醛缩酮反应,(3)4-(λ-羟丙氧基)苯甲醛和乙酰苯或4-(g-羟丙氧基)苯甲醛缩酮反应,(6)4-羟乙氧基苯甲醛-41-溴乙酰苯,(8)4-羟乙氧基苯甲醛缩酮,(12)4-羟乙氧基肉桂酸,(14)羟乙氧基肉桂酸乙酯,(16)4-羟乙氧基苯甲醛-31-硝基乙酰苯,(18)4-羟乙氧基-a-氰基肉桂酸,(19)4-羟乙氧基苯甲醛-41-苯乙酰苯,以及(22)3-氨基苯甲醛缩酮;聚甲基丙烯酸酐与(4)4-(λ-羟丙氧基)苯甲醛和乙酰苯、(10)4-羟乙氧基苯甲醛缩酮和(21)4-(b-羟乙氧基)苯甲醛丙酮酸反应;异丙烯酸酯马来酸酐互缔物与(9)4-羟乙氧基苯甲醛缩酮反应。产品可以溶于溶剂中,例如丙酮、二噁烷、甲乙酮、吡啶、甘醇的单甲醚和单乙醚及其酯,以及氯化烃,并涂覆在支撑物上,例如铝、锌、铜、镁、纸张、表面水解的醋酸纤维素或酪蛋白,并用于摄影和光机械复制。它们可以被敏化,例如与2-苯甲酰亚甲基-1-甲基-b-萘噻唑啉敏化,曝光后,图像可以用溶剂开发,例如稀氨水溶液、水合丙酮或甲乙酮水溶液。 -
Purification, Structural Characterization, and Modification of Organosolv Wheat Straw Lignin作者:Laurie Mbotchak、Clara Le Morvan、Khanh Linh Duong、Brigitte Rousseau、Martine Tessier、Alain FradetDOI:10.1021/acs.jafc.5b02071日期:2015.6.3Deacylated samples with a low content of contaminants were obtained by combining alkaline hydrolysis and solvent extraction. The high phenolic OH content found by 31P NMR reflects the presence of condensed aromatic units, such as 5–5 units. Reaction of purified lignin with ethanol and ethane-1,2-diol yielded esterified lignins much more soluble than Biolignin in common organic solvents. During this reactionBiolignin是一种通过乙酸/甲酸/水水解生产的麦草木质素,其特征在于31 P和13 C– 1 H 2D NMR光谱以及尺寸排阻色谱法。生物木质素是由S,G和H单元以及香豆酸和阿魏酸单元组成的低摩尔质量化合物(M n = 1660 g / mol)的混合物。β-5和β-O-4单元间键在乙酸酯和对-香豆酸酯基团的γ位上被部分酰化。通过将碱性水解和溶剂萃取相结合,可以获得污染物含量低的脱酰样品。31发现高酚羟基含量P NMR反映了稠合的芳族单元(例如5-5个单元)的存在。纯化的木质素与乙醇和乙烷-1,2-二醇的反应生成的酯化木质素比Biolignin在普通有机溶剂中的溶解度高得多。在该反应过程中,β-O-4键的仲OH同时被醚化。由2-氯乙醇进行的苯酚羟乙基化产生的样品仅含有脂肪族羟基。
-
[EN] PROCESS FOR THE PRODUCTION OF ACRYLIC ACID ESTERS CONTAINING CARBOXYL GROUPS<br/>[FR] PROCEDE DE PRODUCTION D'ESTERS D'ACIDE ACRYLIQUE CONTENANT DES GROUPES CARBOXYLE申请人:HUNTSMAN ADV MAT SWITZERLAND公开号:WO2005030696A1公开(公告)日:2005-04-07A process for the production of acrylic esters of general formula (11) by way of reacting a carboxyl group containing hydroxy compound of general formula (a) with acryloyl chloride (c) according to the following general scheme (C): ( II ) wherein A denotes a flexible spacer group, Y a divalent linking group and M a divalent hydrocarbon ring(s) and/or heterocyclic ring(s) containing group containing up to 30 carbon atoms, characterized in that the reaction is carried out a) in the presence of a solvent medium, whereby the solvent medium contains at least 30% per weight based on the total amount of the solvent medium of a solvent selected from the group of N,N-Dimethyl acetamide (DMAc), NMethyl-pyrrolidon (NMP), N,N,N'N'-Tetramethyl urea (TMU) and N-Methylcaprolactam, b) in the absence of a base, and that c) the reaction temperature is kept between -10'C and +50'C; said process is especially suitable for obtaining products in high yield and purity in an industrial scale.
-
Imidazo (1,2-a) Pyridines as bradykinin antagonists申请人:FUJISAWA PHARMACEUTICAL CO., LTD.公开号:EP0596406A1公开(公告)日:1994-05-11A compound of the formula : wherein R1 is halogen, R2 and R3 are each hydrogen, lower alkyl, halo(lower)alkyl or acyl, R4 is aryl having suitable substituent(s), or a heterocyclic group optionally having suitable substituent(s), Q is O or N-R11, in which R11 is hydrogen or acyl, and A is lower alkylene, and pharmaceutically acceptable salts thereof, processes for their preparation and pharmaceutical compositions comprising them as an active ingredient.式中的化合物: 式中 R1 是卤素 R2 和 R3 分别是氢、低级烷基、卤代(低级)烷基或酰基、 R4 是具有合适取代基的芳基,或可选具有合适取代基的杂环基团、 Q 是 O 或 N-R11,其中 R11 是氢或酰基,以及 A 是低级亚烷基,及其药学上可接受的盐、制备工艺和包含它们作为活性成分的药物组合物。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
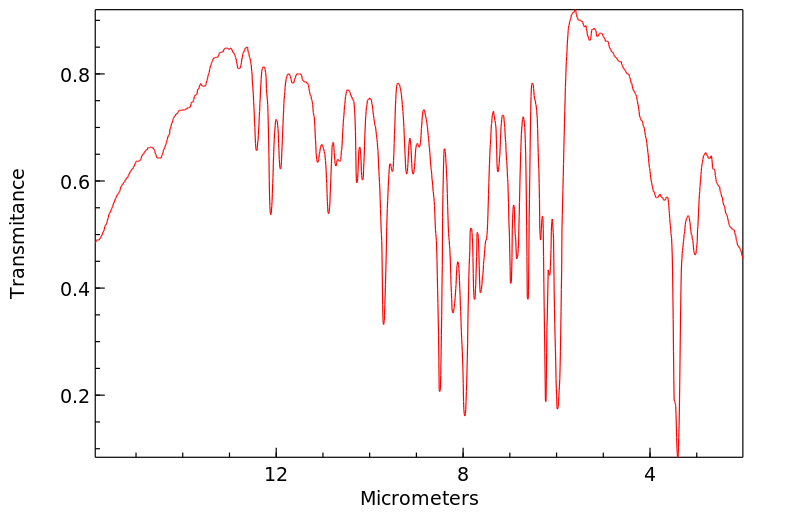
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息