异欧前胡素 | 482-45-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:107-111°C
-
沸点:448.3±45.0 °C(Predicted)
-
密度:1.242±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少量)、乙酸乙酯(少量
-
LogP:3.495 (est)
-
颜色/状态:Off-white, light brown solid
-
蒸汽压力:1.4X10-7 mm Hg at 25 °C (est)
-
稳定性/保质期:
Stable under recommended storage conditions.
-
保留指数:2394
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:20
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:48.7
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:29329990
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:-20°C 冰箱
SDS
制备方法与用途
异欧前胡素的分子式为C16H14O4,分子量270.27。它是一种无色针状结晶(乙醇),熔点为110~111℃。
该化合物具有降压、解痉和抗癌的作用,并可用于治疗银屑病。
羌活中异欧前胡素的含量测定[色谱条件]
[供试品的制备]
取本品粗粉约50g,用8倍量90%乙醇回流提取3次(每次1.5h),合并提取液,回收乙醇,得浸膏13.70g。然后与适量经处理过的的大孔吸附树脂拌匀,置于大孔吸附树脂柱上,依次用水和20%、30%、40%、50%、60%、70%、80%、90%的乙醇冲洗(每次冲至洗脱液无色),水浴蒸干,得膏状物。分别用50%乙醇溶解并定容至50ml。按此法色谱条件进行测定。
生物活性异欧前胡素是当归根甲醇提取物的有效成分之一,在体外对乙酰胆碱酯酶(AChE)表现出抑制作用,其IC50为74.6 μM。
靶点| Target | Value |
|---|---|
| AChE (Cell-free assay) | 74.6 μM |
在一项针对中国药用植物的新农用化学品筛选项目中,发现胡椒科的Notopterygium incisum 块根乙醇提取物对两种线虫Bursaphelenchus xylophilus 和 Meloidogyne incognita 具有强烈的杀线虫活性。通过生物活性导向分离,从该乙醇提取物中分离出四个成分并确认为Columbianetin、Falcarindiol、Falcarinol和异欧前胡素。异欧前胡素对B. xylophilus的LC50值为21.83 μg/mL。
使用15分钟紫外光照射处理,falcarindiol、falcarinol和isoimperatorin对南方根结线虫表现出比暗处理条件下约五倍的毒性。而Columbianetin则仅表现出两倍的毒性。异欧前胡素已显示出对多种昆虫具有杀虫活性,如油菜蚜虫(Brevicoryne brassicae)。它存在于当归提取物中,并通常作为内部标准使用。
化学性质来源:
- 来自大风子科罗旦梅Flacourtia jangomas (Lour.) Raeusch 茎、皮
用途:
- 用于含量测定/鉴定/药理实验等。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 佛手苷内酯 bergapten 484-20-8 C12H8O4 216.193 香柑醇 bergaptol 486-60-2 C11H6O4 202.166 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 水合氧化前胡素; 5-(2,3-二羟基-3-甲基丁氧基)补骨脂素 oxypeucedanin hydrate 24724-52-5 C16H16O6 304.299 氧化前胡素 oxypeucedanin 737-52-0 C16H14O5 286.284 香柑醇 bergaptol 486-60-2 C11H6O4 202.166 —— 6,7-Furano-5-prenyloxy hydrocoumaric acid 1207533-02-5 C16H18O5 290.316 —— 6,7-Furano-5-prenyloxy hydrocoumaric acid methyl ester 1207533-03-6 C17H20O5 304.343
反应信息
-
作为反应物:参考文献:名称:El-Tawil, B.A.H.; Baghlaf, A.O.; Barbood, S.O., Pharmazie, 1980, vol. 35, # 8, p. 503摘要:DOI:
-
作为产物:参考文献:名称:呋喃香豆素单体作为CYP3A4抑制剂的设计,合成和评估。摘要:已发现从葡萄柚汁中分离出的许多呋喃香豆素在体外抑制CYP3A4的活性。在这项研究中,我们设计并合成了一系列基于佛手柑的类似物,以研究化学结构与CYP3A4活性抑制之间的关系。使用人肝微粒体和人肠道S9馏分进行了研究,其中睾丸激素为标记底物。除无活性的香豆素和酚醛呋喃香豆素衍生物外,发现烷氧基-呋喃香豆素类似物以剂量依赖性方式抑制CYP3A4活性,观察到的IC50值范围为0.13 +/- 0.03至49.3 +/- 1.9 microM 。发现不饱和呋喃衍生物表现出时间依赖性抑制,对6',7'的效力显示出2、4和14倍的增加 在预温育十分钟后,分别将-β-环氧香豆素,6',7'-二羟基香柠檬素和香柠檬素。呋喃部分的减少导致抑制力降低11倍,表明该官能团是这些化合物与CYP3A4之间相互作用的关键。DOI:10.1039/b601096b
文献信息
-
Parallel evolution of UbiA superfamily proteins into aromatic <i>O</i> -prenyltransferases in plants作者:Ryosuke Munakata、Alexandre Olry、Tomoya Takemura、Kanade Tatsumi、Takuji Ichino、Cloé Villard、Joji Kageyama、Tetsuya Kurata、Masaru Nakayasu、Florence Jacob、Takao Koeduka、Hirobumi Yamamoto、Eiko Moriyoshi、Tetsuya Matsukawa、Jérémy Grosjean、Célia Krieger、Akifumi Sugiyama、Masaharu Mizutani、Frédéric Bourgaud、Alain Hehn、Kazufumi YazakiDOI:10.1073/pnas.2022294118日期:2021.4.27being beneficial or detrimental to human health. Although their O-prenyl moieties often play crucial roles in the biological activities of these compounds, no plant gene encoding an aromatic O-prenyltransferase (O-PT) has been isolated to date. This study describes the isolation of an aromatic O-PT gene, CpPT1, belonging to the UbiA superfamily, from grapefruit (Citrus × paradisi, Rutaceae). This gene植物产生约 300 种芳香族化合物,它们通过 C-O 键酶促连接到异戊二烯侧链。这些O-异戊二烯化芳香族化合物已在分类学上较远的植物类群中发现,其中一些对人类健康有益或有害。尽管它们的O-异戊二烯基部分通常在这些化合物的生物活性中起关键作用,但迄今为止还没有分离出编码芳香族O-异戊二烯基转移酶 ( O- PT) 的植物基因。该研究描述了一种芳族的隔离Ó -PT基因,CpPT1,属于UbiA超家族,从柚(柑橘×葡萄柚,芸香科)。该基因被证明负责O-异戊二烯化香豆素衍生物的生物合成,这些衍生物会改变药物在人体内的药代动力学。另一个香豆素O -PT 基因编码同一家族的蛋白质,在当归中被鉴定出来,当归是一种含有药学活性的O-异戊二烯化香豆素的药用植物。这些O -PT 的系统发育分析表明,芳香族O -异戊二烯化活性独立于这些远缘植物分类群中的相同祖先基因进化而来。这些发现阐明了通过 UbiA 超家族了解植物次生(特化)代谢物的进化。
-
Natural oxyprenylated coumarins are modulators of melanogenesis作者:Serena Fiorito、Francesco Epifano、Francesca Preziuso、Ivana Cacciatore、Antonio di Stefano、Vito Alessandro Taddeo、Philippe de Medina、Salvatore GenoveseDOI:10.1016/j.ejmech.2018.04.051日期:2018.5Naturally occurring coumarins 7-isopentenyloxycoumarin, auraptene, and umbelliprenin are able to modulate the biosynthesis of melanin in murine Melan-a cells probably through the interaction with selected biological targets like estrogen receptor 13 and aryl hydrocarbon receptor. Such a modulation strictly depends on the individual structure of the coumarin: the presence of a 3,3-dimethylallyloxy side chain is a structural determinant for tanning activation whereas a farnesyl one leads to the opposite effect. The parent compound with a free OH group, umbelliferone, did not provide any interaction. Other coumarins assayed, having shorter chains and/or being substituted in other positions, and prenyloxypsoralens, were not active or not further investigated in this context being cytotoxic at low doses. (C) 2018 Elsevier Masson SAS. All rights reserved.
-
Resolution of prenyl bromohydrin esters and derivatives: synthesis of the quinoline alkaloid (+)-(R)- and (−)-(S)- lunacridine作者:Stephen A. Barr、Derek R. Boyd、Narain D. Sharma、Timothy A. Evans、John F. Malone、Vimal D. MehtaDOI:10.1016/s0040-4020(01)89424-2日期:1994.1Chromatographic separation of the bromohydrin MTPA diastereoisomers formed at the prenyl group attached to quinoline and coumarin rings is reported; base catalysed cyclization of the bromo MTPA esters in the quinoline series yielded the corresponding prenyl epoxide enantiomers. This provides a synthetic route to the enantiopure quinoline alkaloid lunacridine 11 and to the dihydrofuroquinoline 8-methoxy-platydesmine 8.
-
Biotransformation of isoimperatorin and imperatorin by Glomerella cingulata and β-secretase inhibitory activity作者:Shinsuke Marumoto、Mitsuo MiyazawaDOI:10.1016/j.bmc.2009.10.004日期:2010.1Biotransformation studies conducted on the furanocoumarins isoimperatorin (1) and imperatorin (3) have revealed that 1 was metabolized by Glomerella cingulata to give the corresponding reduced acid, 6,7-furano-5-prenyloxy hydrocoumaric acid (2), and 3 was transformed by G. cingulata to give the dealkylated metabolite, xanthotoxol (4) in high yields (83% and 81%), respectively. The structures of the new compound 2 have been established on the basis of spectral data. The metabolites 2 and 4 were tested for the beta-secretase (BACE1) inhibitory activity in vitro, and metabolite 2 slightly inhibited the beta-secretase activity with an IC50 value of 185.6 +/- 6.8 mu M. The metabolite 4 was less potent activity than compounds 1-3. In addition, methyl ester (2Me), methyl ether (2a) and methyl ester and ether (2aMe) of 2 were synthesized, and investigated for the ability to inhibit beta-secretase. Compound 2aMe exhibited the best beta-secretase inhibitory activity at the IC50 value 16.2 +/- 1.2 mu M and found to be the 2aMe showed competitive mode of inhibition against beta-secretase with K-i value 11.3 +/- 2.8 mu M. (C) 2009 Elsevier Ltd. All rights reserved.
-
Chakraborti; Bose, Transactions of the Bose Research Institute (Calcutta), 23 <1959/60) 55作者:Chakraborti、BoseDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
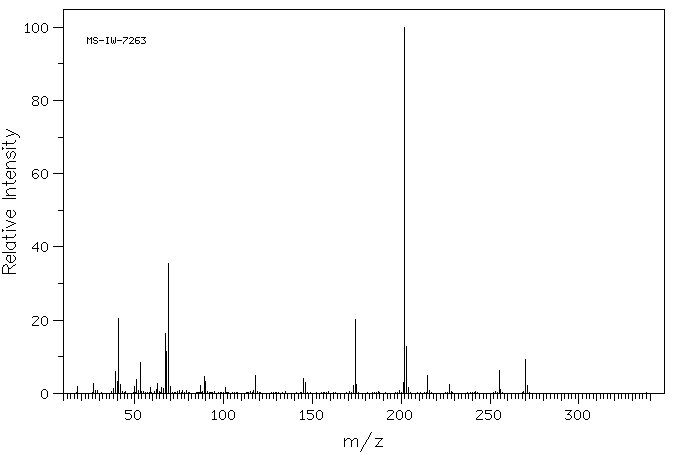
质谱MS
-
碳谱13CNMR
-
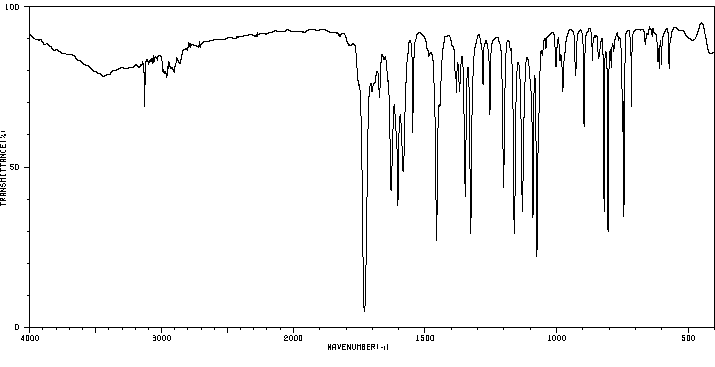
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息