吡啶并[3,4-D]嘧啶-2,4(1H,3H)-二酮 | 21038-67-5
中文名称
吡啶并[3,4-D]嘧啶-2,4(1H,3H)-二酮
中文别名
——
英文名称
pyrido[3,4-d]pyrimidine-2,4(1H,3H)-dione
英文别名
1,2,3,4-Tetrahydro-2,4-diazo-pyrido<3.4-d>pyrimidin;1H-pyrido[3,4-d]pyrimidine-2,4-dione
CAS
21038-67-5
化学式
C7H5N3O2
mdl
MFCD07438024
分子量
163.136
InChiKey
MNNWQAIAFUMNOS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>320 °C
-
密度:1.424±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:71.1
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2933990090
-
储存条件:室温且干燥
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Pyrido[3,4-d]pyrimidine-2,4(1h,3h)-dione
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Pyrido[3,4-d]pyrimidine-2,4(1h,3h)-dione
CAS number: 21038-67-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H5N3O2
Molecular weight: 163.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Pyrido[3,4-d]pyrimidine-2,4(1h,3h)-dione
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Pyrido[3,4-d]pyrimidine-2,4(1h,3h)-dione
CAS number: 21038-67-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H5N3O2
Molecular weight: 163.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:吡啶并[3,4-D]嘧啶-2,4(1H,3H)-二酮 在 N,N-二异丙基乙胺 、 三氯氧磷 作用下, 以 二甲基亚砜 、 甲苯 为溶剂, 反应 22.33h, 生成 2-chloro-N-methyl-N-(1,1,1-trifluoropropan-2-yl)pyrido[3,4-d]pyrimidin-4-amine参考文献:名称:METHODS AND COMPOSITIONS FOR OCULAR CELL THERAPY摘要:本发明提供了通过CRISPR系统靶向B2M表达进行基因修饰的眼部细胞,用于眼部细胞治疗。该发明进一步提供了生成扩增的基因修饰眼部细胞群体的方法,例如角膜边缘干细胞(LSCs)或角膜内皮细胞(CECs),其中细胞的扩增涉及使用LATS抑制剂,并且细胞中的B2M表达已经降低或消除。本发明还提供了细胞群体、制剂、用途和包括所述细胞的治疗方法。公开号:US20200131474A1
-
作为产物:描述:参考文献:名称:四乙酸铅的反应。第三部分 由二羧酸酰胺形成嘧啶离子和相关化合物摘要:用四乙酸铅在二甲基甲酰胺中处理邻苯二甲酰胺(X),得到1,2,3,4-四氢-2,4-二氧代喹唑啉(XI)。吡啶-2,3-和-3,4-二甲酰胺,琥珀酰胺,2-苯基琥珀酰胺和合适的N-单烷基二酰胺经历类似的环化反应。当用四乙酸铅加热时,邻氨基苯甲酸(Ⅰ)和2-氨基甲酰基烟酸(Ⅵ)可产生适当的肟类衍生物(Ⅲ)和(VII),但用2-苯基琥珀酰胺酸(VIII)则该方法失败。这些转变的机制似乎涉及异氰酸酯的初始形成。DOI:10.1039/j39680002756
文献信息
-
One-Step Synthesis of 2-Chloropyrimidin-4-ol Derivatives: An Unusual Reactivity of Thiophosgene作者:Michael Callingham、Francesca Blum、Grégoire PavéDOI:10.1021/acs.orglett.5b02375日期:2015.10.2A novel, high-yielding, one-step synthesis of 2-chloroquinazolin-4-ols and analogous bicycles from 2-aminoamides using thiophosgene is described. The scope of the reaction includes aminothioamides, amino acids, and fused heterocycle derivatives, furnishing quinazolines, oxazinones, and substituted fused pyrimidine bicycles, respectively. On the basis of observed results with substituted analogues,
-
[EN] GLUCOSE UPTAKE INHIBITORS<br/>[FR] INHIBITEURS D'ABSORPTION DU GLUCOSE申请人:KADMON CORP LLC公开号:WO2016210330A1公开(公告)日:2016-12-29Provided hererin are compounds that modulate glucose uptake activityand are useful for treating cancer, autoimmune diseases, inflammation, infectious diseases, and metabolic diseases. In certain embodiments, the compounds modulate glucose uptake activity by modulating cellular components, including, but not limited to those related to glycolysis and known transporters/co-transporters of glucose such as GLUT1 and other GLUT family members/alternative hexose transporters. In certain embodiments, the compounds have the structure of formula I: Formula (I) wherein the variables have the values disclosed herein.
-
Azaisatoic anhydrides申请人:The Sherwin-Williams Company公开号:US03947416A1公开(公告)日:1976-03-30A method for producing heterocyclic acid anhydrides and pyrimidinediones from the corresponding acids, dicarboxamides, 2,3-and 3,4-pyridinedicarboxamides, and N-monosubstituted 2,3-and 3,4-pyridinedicarboxamides, in which the aforesaid compounds are reacted with lead tetra-acetate in the presence of a suitable anhydrous inert solvent.
-
[EN] HISTONE DEMETHYLASE INHIBITORS<br/>[FR] INHIBITEURS D'HISTONE DÉMÉTHYLASE申请人:QUANTICEL PHARMACEUTICALS INC公开号:WO2014151106A1公开(公告)日:2014-09-25The present invention relates generally to compositions and methods for treating cancer and neoplastic disease. Provided herein are substituted pyrido[3,4-d]pyrimidin-4-one derivative compounds and pharmaceutical compositions comprising said compounds. The subject compounds and compositions are useful for inhibition of histone demethylase. Furthermore, the subject compounds and compositions are useful for the treatment of cancer, such as prostate cancer, breast cancer, bladder cancer, lung cancer and/or melanoma and the like.
-
杂环并嘧啶二酮类化合物制备方法
表征谱图
-
氢谱1HNMR
-
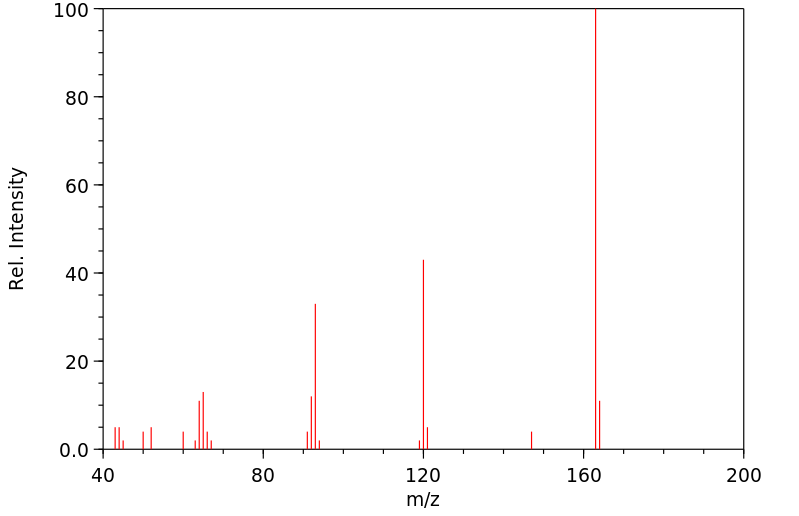
质谱MS
-
碳谱13CNMR
-
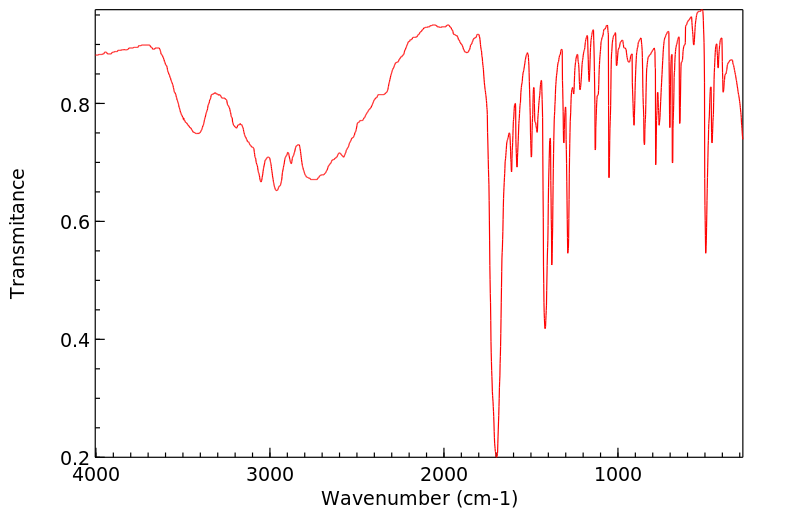
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿昔替酯
螺喹唑啉
苯并[g][1,2,3]三唑并[4',5':5,6]吡啶并[2,1-b]喹唑啉-13(2H)-酮
脱氢利培酮
盐酸曲林菌素
甲硫利马唑
甲基8-乙基-2-甲氧基-5-氧代-5,8-二氢吡啶并[2,3-d]嘧啶-6-羧酸酯
甲基8-乙基-2-(甲硫基)-5-氧代-5,6,7,8-四氢吡啶并[2,3-d]嘧啶-6-羧酸酯
甲基2-乙氧基-8-乙基-5-氧代-吡啶并[6,5-d]嘧啶-6-羧酸酯
溴他替尼
泮托拉唑杂质DF
氨甲酸,[(2R,3E)-2-羟基-3-戊烯基]-,1,1-二甲基乙基酯(9CI)
柱孢藻毒素
曲美替尼
曲美替尼
曲喹辛
异噻唑并[5,4-d]嘧啶,3-亚硝基-(9CI)
帕潘立酮棕榈酸酯
帕潘立酮杂质7
帕潘立酮杂质16
帕潘立酮杂质
帕潘立酮杂质
帕潘立酮去氟杂质
帕潘立酮Z-异构体
帕潘立酮
帕泊昔布杂质117
帕布昔利布杂质46
帕博西尼杂质S
帕利哌酮杂质05
帕利哌酮杂质03
帕利哌酮杂质02
帕利哌酮十四酸酯
帕利哌酮N-氧化物
布喹特林
巴马斯汀
奥卡哌酮
多夸司特
嘧啶并[4,5-d]嘧啶-2,4,5(1H,3H,6H)-三酮,7-乙氧基-1,3-二甲基-6-(苯基甲基)-
吡曲克辛
吡嘧司特钾
吡嘧司特
吡啶并[4,3-d]嘧啶-4(1H)-酮,4,5,6,7-四氢-6-甲基-2-苯基-
吡啶并[4,3-D]嘧啶-2,4(1H,3H)-二酮
吡啶并[3,4-D]嘧啶-2,4(1H,3H)-二酮
吡啶并[3,2-d]嘧啶-4(3H)-酮,3-甲基-2-(甲基氨基)-
吡啶并[3,2-d]嘧啶-4(3H)-酮
吡啶并[3,2-d]嘧啶-4(1H)-酮,2,3-二氢-3-(2-羟基苯基)-2-硫代-
吡啶并[3,2-d]嘧啶-2,4(1H,3H)-二酮
吡啶并[2,3-d]嘧啶-7(8h)-酮,2,6-二溴-8-环戊基-5-甲基-
吡啶并[2,3-d]嘧啶-7(8H)-酮