四-O-乙酰基-β-D-吡喃核糖 | 4049-34-7
中文名称
四-O-乙酰基-β-D-吡喃核糖
中文别名
四-O-乙酰基-Β-D-吡喃核糖;β-D-吡喃核糖-1,2,3,4-四乙酸酯;四-O-乙酰基-b-D-吡喃核糖
英文名称
1,2,3,4-tetra-O-acetyl-β-D-ribopyranose
英文别名
Tetra-O-acetyl-beta-D-ribopyranose;[(3R,4R,5R,6S)-4,5,6-triacetyloxyoxan-3-yl] acetate
CAS
4049-34-7
化学式
C13H18O9
mdl
——
分子量
318.281
InChiKey
MJOQJPYNENPSSS-LPWJVIDDSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:110-112 °C(lit.)
-
沸点:362.9±42.0 °C(Predicted)
-
密度:1.29±0.1 g/cm3(Predicted)
-
溶解度:在甲醇中几乎透明
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:22
-
可旋转键数:8
-
环数:1.0
-
sp3杂化的碳原子比例:0.69
-
拓扑面积:114
-
氢给体数:0
-
氢受体数:9
安全信息
-
WGK Germany:3
-
海关编码:2932999099
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,3,4-tri-O-acetyl-β-D-ribopyranose —— C11H16O8 276.243
反应信息
-
作为反应物:描述:四-O-乙酰基-β-D-吡喃核糖 在 氢溴酸 、 氰化汞 、 溶剂黄146 作用下, 以 乙腈 为溶剂, 反应 26.0h, 生成 1-(2,3,4-tri-O-acetyl-β-D-ribopyranosyl)-2-nitroimidazole参考文献:名称:碘化阿霉素核苷类组织缺氧新标志物的合成和放射性标记。摘要:已经合成了七种二代碘化阿霉素核苷类缺氧标记物,并在体外和体内测试了肿瘤细胞的缺氧标记活性。β-D-碘阿唑霉素半乳糖苷 (IAZG) 和 β-D-碘阿霉素木吡喃糖苷 (IAZXP) 表现出优于 IAZA 的缺氧标记特性,因为它们具有更高的水溶性、从荷瘤小鼠体内快速清除血浆的速度和最大肿瘤/血液 ( /B) 和肿瘤/肌肉 (T/M) 比率。我们对动物肿瘤模型的研究表明,通过闪烁扫描或平面成像确定的这些标志物的 T/B 或 T/M 比率可以预测肿瘤缺氧的相对程度和肿瘤的放射抗性。© 1997 约翰威利父子公司。DOI:10.1002/(sici)1099-1344(199707)39:7<541::aid-jlcr5>3.0.co;2-b
-
作为产物:参考文献:名称:The Acid-catalyzed Anomerization of Acetylated Aldopyranoses1摘要:DOI:10.1021/ja01515a041
文献信息
-
Synthesis of Theophylline and 6-Thiotheophylline 7-Ribosyl Nucleosides作者:Rodrigo Rico-Gómez、Angel Rodríguez-González、Josefa Ríos-Ruíz、Francisco Nájera、J. Manuel López-RomeroDOI:10.1002/ejoc.200300377日期:2003.10The syntheses of theophylline and thiotheophylline 7-ribopyranose and 7-ribofuranose nucleosides, both by direct coupling of the base and the sugar and by construction of the imidazole ring, are reported. The conformational syn/anti equilibrium of the peracetyl derivatives was studied by molecular mechanics and by the low-temperature line-shape/1H NMR method. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451
-
The influence of boric acid on the acetylation of aldoses: ‘one-pot’ syntheses of penta-O-acetyl-β-D-glucofuranose and its crystalline propanoyl analogue作者:Richard H. Furneaux、Phillip M. Rendle、Ian M. SimsDOI:10.1039/b002841j日期:——When glucose and boric acid are heated in acetic acid a soluble compound forms from which, with acetic anhydride and catalytic amounts of sulfuric acid, a mixture consisting of >90% of the glucofuranose per-acetates (αâ¶Î² ratio 1â¶1.8) is obtained in high yield. In the absence of the sulfuric acid partial acetylation takes place and penta-O-acetyl-β-D-glucofuranose (αâ¶Î² ratio 1â¶52) is obtainable in good yield by removal of boric acid and completion of the esterification by addition of acetic anhydride and pyridine. A new, crystalline glucofuranose, penta-O-propanoyl-β-D-glucofuranose, is obtained in 58% yield in a âone-potâ procedure using boric acid. The effects of boric acid on the acid-catalysed acetylation of other aldo-hexoses and -pentoses suggest that the synthesis of furanosyl per-esters could be successful with xylose and idose as well as with glucose. p
-
An improved method for the synthesis of anhydroalditols作者:Arlen Jeffery、Vasu NairDOI:10.1016/0040-4039(95)00618-m日期:1995.5An improved synthesis of anhydroalditols through reductive cleavage of acetylated carbohydrates is reported. The procedure appears to have generality. Ring rearrangements, which complicate some other reported procedures for the preparation of anhydroalditols, were not observed. The acetate protecting groups used were stable under the reaction conditions employed.
-
Convenient syntheses of symmetrical and unsymmetrical glycosyl carbodiimides and N,N-bis(glycosyl)cyanamides作者:László Kovács、Erzsébet Ősz、Zoltán GyörgydeákDOI:10.1016/s0008-6215(02)00108-8日期:2002.7carbon disulfide under mild conditions (room temperature, short reaction time) leads to symmetrical glycosyl carbodiimides. Addition of bis(trimethylsilyl)carbodiimide to peracetylated aldoses under the influence of SnCl(4) afforded N,N-bis(glycosyl)cyanamides for the first time. Readily accessible unsymmetrical N,N'-bis(glycosyl)thioureas can be desulfurated and transformed into the corresponding carbodiimides
-
Sugar-Pendant Diamines作者:Yuji Mikata、Yoshie Shinohara、Kazumi Yoneda、Yuka Nakamura、Kimiko Esaki、Maki Tanahashi、Izabela Brudziñska、Shiho Hirohara、Mika Yokoyama、Kaoru Mogami、Tomoaki Tanase、Takashi Kitayama、Katsuki Takashiba、Kenji Nabeshima、Rie Takagi、Masahiko Takatani、Tadashi Okamoto、Isamu Kinoshita、Matsumi Doe、Akihisa Hamazawa、Masanori Morita、Fumiko Nishida、Toru Sakakibara、Chris Orvig、Shigenobu YanoDOI:10.1021/jo001702+日期:2001.6.1A set of 1,3-propanediamine derivatives connected to carbohydrates (5) has been prepared in four steps from peracetylated sugar and 1,3-dibromo-2-propanol in 60-73% yields. D-Glucose, D-mannose, D-galactose, D-xylose, D-ribose, and maltose are utilized as sugar molecules in this work. The diamine moiety was connected to the C1 carbon of the glycopyranose ring via an O-glycoside bond. All of the anomeric分四个步骤从过乙酰化糖和1,3-二溴-2-丙醇中以60-73%的收率制备了一组与碳水化合物(5)连接的1,3-丙二胺衍生物。在这项工作中,D-葡萄糖,D-甘露糖,D-半乳糖,D-木糖,D-核糖和麦芽糖被用作糖分子。二胺部分通过O-糖苷键连接到吡喃吡喃糖环的C 1碳上。通过Diadodo或dibromo前体的X射线晶体学测定,除了D-麦芽糖衍生物中的所有异头构型和糖起皱构象。尽管在三氟化硼存在下用1,3-二溴-2-丙醇对过乙酰化的吡喃半乳糖进行糖基化可提供两种端基异构体,但2-乙酰氧基的相邻基团参与可为其他底物产生单一的端基异构体。该方法已经用于合成糖悬垂二胺的文库,其包括OH保护的衍生物(6)和N,N′-二异丙基取代的衍生物(7)。使用2,3-二溴-1-丙醇的一系列类似反应生成乙二胺型衍生物(11),双(溴甲基)双(羟甲基)甲烷(12)生成双葡萄糖侧链衍生物(16)。
表征谱图
-
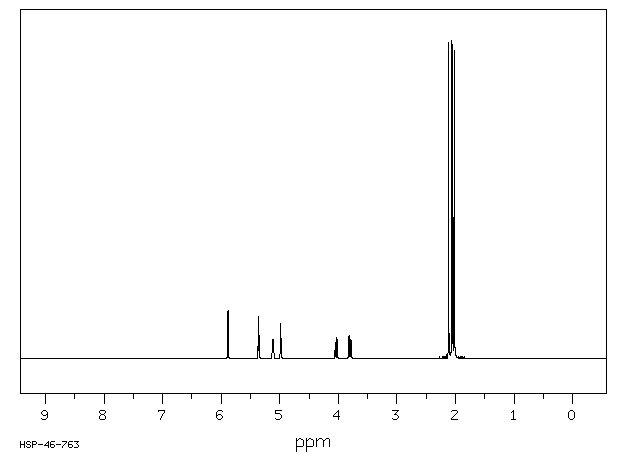
氢谱1HNMR
-
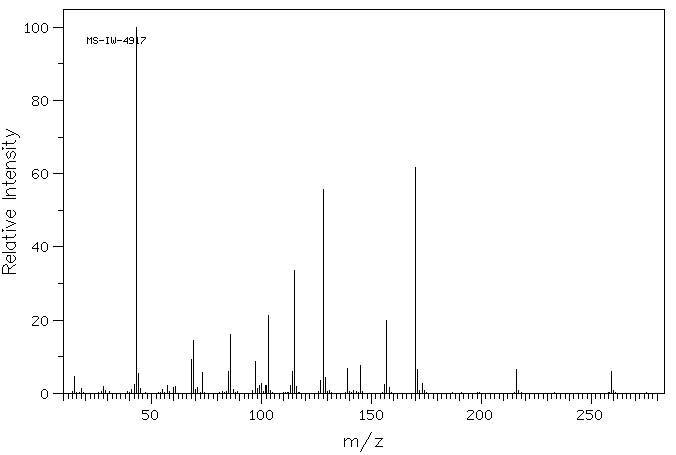
质谱MS
-
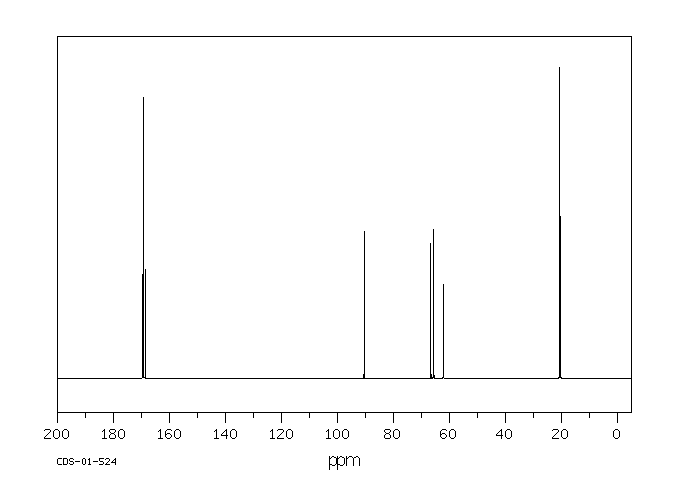
碳谱13CNMR
-
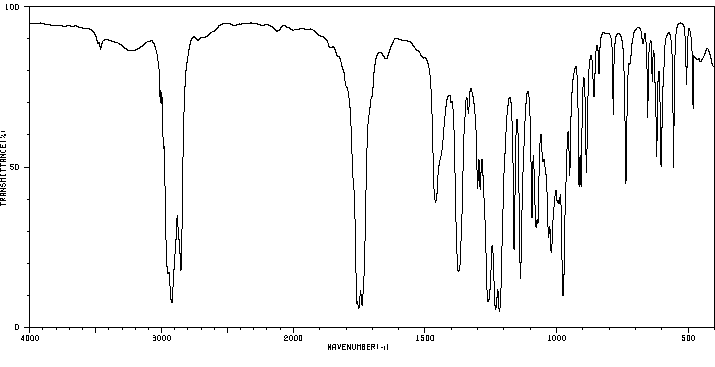
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸