1-苄基-1H-咪唑-2-甲醛 | 10045-65-5
中文名称
1-苄基-1H-咪唑-2-甲醛
中文别名
1H-2-咪唑甲醛,1-(苯基甲基)-;1-(苄基)咪唑-2-甲醛;1-(苯基甲基)-2-咪唑甲醛;2-咪唑甲醛,1-苄基-;1-苄基2-咪唑甲醛;1-苄基-2-甲酰咪唑;1-(苯基甲基)咪唑-2-甲醛
英文名称
N-benzylimidazole-2-carboxaldehyde
英文别名
1-benzylimidazole-2-carboxaldehyde;1-benzyl-2-imidazolylcarboxaldehyde;1-Benzyl-1H-imidazole-2-carbaldehyde;1-benzylimidazole-2-carbaldehyde
CAS
10045-65-5
化学式
C11H10N2O
mdl
MFCD00088667
分子量
186.213
InChiKey
AKFHFMMKMUJLBU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:128 °C(Press: 4 Torr)
-
密度:1.12±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:34.9
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
海关编码:2933290090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-苯并噻吩-5-基异氰酸酯 1-benzyl-2-(hydroxymethyl)imidazole 5376-10-3 C11H12N2O 188.229 N-苄基咪唑 1-benzylimidazole 4238-71-5 C10H10N2 158.203 1-苄基-1H-咪唑-2-硫醇 1-benzylimidazole-2-thione 23269-10-5 C10H10N2S 190.269 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苄基-2-咪唑羧酸 1-benzyl-1H-imidazole-2-carboxylic acid 16042-26-5 C11H10N2O2 202.213 1-苯并噻吩-5-基异氰酸酯 1-benzyl-2-(hydroxymethyl)imidazole 5376-10-3 C11H12N2O 188.229 (1-苄基-1H-咪唑-2-基)甲基胺 (1-benzylimidazol-2-yl)methylamine 26163-58-6 C11H13N3 187.244 —— (NE)-N-[(1-benzylimidazol-2-yl)methylidene]hydroxylamine —— C11H11N3O 201.22 —— N-[(E)-(1-benzylimidazol-2-yl)methylideneamino]-N-methylmethanamine —— C13H16N4 228.29 —— 1-benzyl-1H-imidazole-2-carbaldehyde thiosemicarbazone 20062-64-0 C12H13N5S 259.335 —— 1-benzyl-2-[1,3]dioxolan-2-yl-1H-imidazole 25603-17-2 C13H14N2O2 230.266
反应信息
-
作为反应物:描述:参考文献:名称:Some benzyl-substituted imidazoles, triazoles, tetrazoles, pyridinethiones, and structural relatives as multisubstrate inhibitors of dopamine .beta.-hydroxylase. 4. Structure-activity relationships at the copper binding site摘要:Structure-activity relationships (SAR) were determined for novel multisubstrate inhibitors of dopamine beta-hydroxylase (DBH; EC 1.14.17.1) by examining the effects upon in vitro inhibitory potencies resulting from structural changes at the copper-binding region of inhibitor. Attempts were made to determine replacement groups for the thione sulfur atom of the prototypical inhibitor 1-(4-hydroxybenzyl)imidazole-2-thione described previously. The synthesis and evaluation of oxygen and nitrogen analogues of the soft thione group demonstrated the sulfur atom to be necessary for optimal activity. An additional series of imidazole-2-thione relatives was prepared in an effort to probe the relationship between the pKa of the ligand group and inhibitory potency. In vitro inhibitory potency was shown not to correlate with ligand pKa over a range of approximately 10 pKa units, and a rationale for this is advanced. Additional ligand modifications were prepared in order to explore bulk tolerance at the enzyme oxygen binding site and to determine the effects of substituting a six-membered ligand group for the five-membered imidazole-2-thione ligand.DOI:10.1021/jm00164a051
-
作为产物:描述:参考文献:名称:利用手性C1对称二氮配体的不对称铜(II)催化的硝基醛(亨利)反应摘要:通过手性胺与各种取代的咪唑甲醛的缩合,可以方便地以高收率合成一系列手性C 1对称二氮配体。配体L1与CuCl 2 · 2H 2 O结合可以高产率(高达97%)和良好的对映选择性(高达96%)有效地促进各种醛与硝基甲烷之间的硝基醛(亨利)反应。DOI:10.1002/ejoc.201100857
文献信息
-
1-(Aromatic- or heteroaromatic-substituted)-3-(heteroaromatic substituted)-1,3-propanediones and uses thereof申请人:——公开号:US20030229079A1公开(公告)日:2003-12-11Certain 1-(aromatic- or heteroaromatic-substituted-3-(heteroaromatic substituted)-1,3-propanediones are described as inhibitors of HIV integrase and inhibitors of HIV replication. These compounds are useful in the prevention or treatment of infection by HIV and the treatment of AIDS, either as compounds, pharmaceutically acceptable salts, pharmaceutical composition ingredients, whether or not in combination with other antivirals, immunomodulators, antibiotics or vaccines. Methods of treating AIDS and methods of preventing or treating infection by HIV are also described.
-
Substituted heterocyclylisoquinolinium salts and compositions and method申请人:Sterling Winthrop Inc.公开号:US05569655A1公开(公告)日:1996-10-29Substitutued heterocyclylisoquinolinium salts, pharmaceutical compositions containing them and methods for the treatment or prevention of neurodegenerative disorders or neurotoxic injuries utilizing them.取代杂环异喹啉盐,含有它们的药物组合物以及利用它们治疗或预防神经退行性疾病或神经毒性损伤的方法。
-
Selection of Chiral Zinc Catalysts for the Kinetic Resolution of Esters via Dynamic Templating作者:R. Kannappan、K. M. NicholasDOI:10.1021/co3001023日期:2013.2.11of chiral tetradentate bis-imine zinc(II) complexes have been prepared and screened for (1) their discrimination of enantiomeric picolinate esters and pyridyl phosphonate transition state analogs (TSAs) and (2) their catalytic activity and selectivity for enantioselective methanolysis of racemic picolinate esters. The zinc complexes are in equilibrium with their imine ligands as well as with the aldehyde制备并筛选了手性四齿双亚胺锌(II)配合物的动态组合库,用于(1)鉴别对映体吡啶甲酸酯和吡啶基膦酸酯过渡态类似物(TSA),以及(2)催化活性和对映选择性甲醇分解的选择性外消旋吡啶甲酸酯。锌配合物与其亚胺配体以及形成它们的醛和胺结构单元处于平衡状态,从而使文库的组成能够适应配位底物或TSA的引入。二进制(L)的Zn(OTF)(SOLV)+络合物单独地或在由手性酒石酸盐衍生的二胺(库产生的2,3)和一组N-杂环醛(4 – 12)和通过ESI-MS分析建立的配合物分布。(diimine)Zn(OTf)2复杂文库与对映异构体R-和S -2-吡啶基膦酸酯TSA 13的结合研究表明,通过形成对映异构体比率低至中等的非对映异构体LZn(R / S - 13)+络合物形成手性区分,k R / k S(α),范围从0.5到5.0;选定的二元配合物与对映体底物PyrCO 2 CH(OH)Ph(1的相应模板),手性识别率可忽略不计。几种L
-
Manganese-Catalyzed Enantioselective Hydrogenation of Simple Ketones Using an Imidazole-Based Chiral PNN Tridentate Ligand作者:Fei Ling、Jiachen Chen、Sanfei Nian、Huacui Hou、Xiao Yi、Feifei Wu、Min Xu、Weihui ZhongDOI:10.1055/s-0039-1690783日期:2020.2A series of Mn(I) catalysts containing imidazole-based chiral PNN tridentate ligands with controllable ‘side arm’ groups have been established, enabling the inexpensive base-promoted asymmetric hydrogenation of simple ketones with outstanding activities (up to 8200 TON) and good enantioselectivities (up to 88.5% ee). This protocol features wide substrate scope and functional group tolerance, thereby
-
[EN] INDOLIZINE DERIVATIVES WITH CRTH2 RECEPTOR AFFINITY FOR THE TREATMENT OF INFLAMMATORY DISEASES<br/>[FR] DÉRIVÉS D'INDOLIZINE AVEC UNE AFFINITÉ POUR LE RÉCEPTEUR CRTH2 DESTINÉS AU TRAITEMENT DE MALADIES INFLAMMATOIRES申请人:ARGENTA DISCOVERY LTD公开号:WO2009044147A1公开(公告)日:2009-04-09Compounds of formula (I) are ligands of the CRTH2 receptor, useful in the treatment of, for example, inflammatory respiratory disease: R1 is fluoro, chloro, CN or CF3; R2 is hydrogen, fluoro or chloro; X is -CH2-, or - S(O)n-; Ar1 is phenyl or 5- or 6-membered heteroaryl, wherein the phenyl or heteroaryl rings are optionally substituted with one substituent selected from fluoro, chloro, CN, -O(C1-C3alkyi) or C1-C3alkyl, the latter two groups being optionally substituted by one or more fluoro atoms; Y is -CR3R4-, -C(O)-, -O-, -S(O)2- or -NR3-; R3 and R4 independently represent hydrogen or C1-C3alkyl; Ar2 is phenyl or 5- or 6- membered heteroaryl, wherein the phenyl or heteroaryl rings are optionally substituted by one or more substituents independently selected from halogen, - CN, -S(O)nR5, -S(O)2NR6R7, -NR6S(O)2R5 -NR6R7, -NR6COR5, -CONR6R7, -COR5, -OR6, C1- C6alkyl or C3-C7cycloalkyl, the latter two groups being optionally substituted by one or more fluoro atoms; R5 is C1-C6alkyl or C3-C7cycloalkyl, optionally substituted by one or more fluoro atoms; R6 and R7 independently represent hydrogen, C1-C6alkyl or C3-C7cycloalkyl, the latter two groups being optionally substituted by one or more fluoro atoms; and n is O, 1 or 2.化合物的结构式(I)是CRTH2受体的配体,在治疗例如炎症性呼吸道疾病方面很有用:R1是氟、氯、CN或CF3;R2是氢、氟或氯;X是-CH2-或-S(O)n-;Ar1是苯基或5-或6-成员杂环基,其中苯基或杂环基环可以选择性地用来自氟、氯、CN、-O(C1-C3烷基)或C1-C3烷基的一个取代基取代,后两个基可以选择性地用一个或多个氟原子取代;Y是-CR3R4-,-C(O)-,-O-,-S(O)2-或-NR3-;R3和R4独立地代表氢或C1-C3烷基;Ar2是苯基或5-或6-成员杂环基,其中苯基或杂环基环可以选择性地用一个或多个取代基独立地选自卤素、-CN、-S(O)nR5、-S(O)2NR6R7、-NR6S(O)2R5、-NR6R7、-NR6COR5、-CONR6R7、-COR5、-OR6、C1-C6烷基或C3-C7环烷基取代,后两个基可以选择性地用一个或多个氟原子取代;R5是C1-C6烷基或C3-C7环烷基,可以选择性地用一个或多个氟原子取代;R6和R7独立地代表氢、C1-C6烷基或C3-C7环烷基,后两个基可以选择性地用一个或多个氟原子取代;n是O、1或2。
表征谱图
-
氢谱1HNMR
-
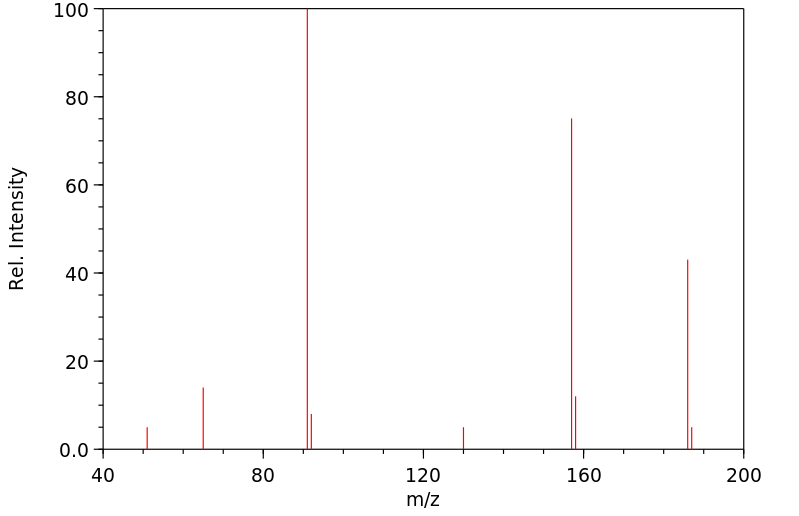
质谱MS
-
碳谱13CNMR
-
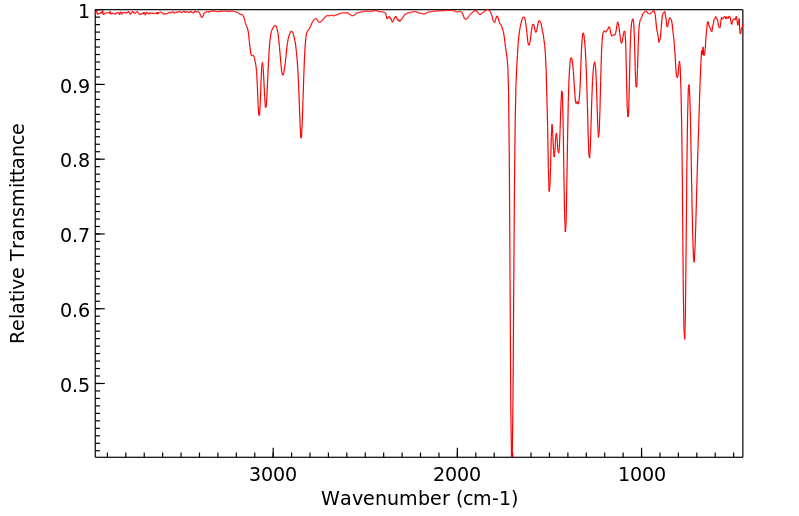
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷