3-methylbutyl (2E)-3-phenyl-2-propenoate | 85180-66-1
中文名称
——
中文别名
——
英文名称
3-methylbutyl (2E)-3-phenyl-2-propenoate
英文别名
3-methylbutyl cinnamate;isopentyl cinnamate;isoamyl cinnamate;trans-cinnamic acid isopentyl ester;trans-Zimtsaeure-isopentylester;3-methylbutyl (E)-3-phenylprop-2-enoate
CAS
85180-66-1
化学式
C14H18O2
mdl
MFCD00026518
分子量
218.296
InChiKey
JFHCDEYLWGVZMX-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133 °C(Solv: methanol (67-56-1))
-
沸点:216 °C
-
密度:1.006±0.06 g/cm3(Predicted)
-
LogP:4.340 (est)
-
物理描述:Isoamyl cinnamate is a pale yellow liquid with iridescent sheen. (NTP, 1992)
-
闪点:greater than 212 °F (NTP, 1992)
-
溶解度:less than 1 mg/mL at 68° F (NTP, 1992)
-
折光率:1.533-1.541
-
保留指数:1717.6;1719
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:16
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.357
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
上下游信息
反应信息
-
作为产物:描述:反式肉桂醛 、 异戊醇 在 amberlyst-15 、 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 甲苯 为溶剂, 反应 0.67h, 生成 3-methylbutyl (2E)-3-phenyl-2-propenoate参考文献:名称:An efficient chemoselective strategy for the preparation of (E)-cinnamic esters from cinnamaldehydes using heterogeneous catalyst and DDQ摘要:An efficient chemoselective protocol is developed for the synthesis of (E)-cinnamic esters from substituted cinnamaldehydes or cinnamyl alcohols using a combination of DDQ and heterogeneous catalyst under microwave irradiation. The method showed remarkable selectivity for cinnamaldehydes over aliphatic and aromatic aldehydes, which is a novel finding. The results demonstrate that the developed protocol can be a useful synthetic tool for chemoselective esterification in total synthesis of complex organic compounds. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2006.11.011
文献信息
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
Kinetics of trans-Cinnamic Anhydride Reactions Catalyzed by Pyridine, 4-Dimethylaminopyridine, and N-Methylimidazole作者:Shu-Fen Lin、Kenneth A. ConnorsxDOI:10.1002/jps.2600700302日期:1981.3pyridine, 4-dimethylaminopyridine, and N-methylimidazole as catalysts. The absolute rates of the catalyzed hydrolysis decreased with increasing acetonitrile content (decreasing solvent polarity), but the catalytic efficiency of N-methylimidazole and 4-dimethylaminopyridine relative to pyridine increased as the solvent polarity decreased. The relative catalytic rates for the cinnamoylation of n-propanol
-
Design, Synthesis, and Preliminary Evaluation of Substituted Cinnamic Acid Esters as Selective Matrix Metalloproteinase Inhibitors作者:Zhi-Hao Shi、Nian-Guang Li、Qian-Ping Shi、Hao-Tang、Yu-Ping TangDOI:10.1002/ddr.21015日期:2012.9Strategy, Management and Health Policy Preclinical Research战略,管理与卫生政策 临床前研究
-
Esters, amides and substituted derivatives of cinnamic acid: synthesis, antimicrobial activity and QSAR investigations作者:Balasubramanian Narasimhan、Deepak Belsare、Devayani Pharande、Vishnukant Mourya、Avinash DhakeDOI:10.1016/j.ejmech.2004.06.013日期:2004.10A series of esters (I(a-k)), substituted derivatives (II(a-d)) and amides (III(a-q)) of cinnamic acid were synthesized and evaluated as antibacterial and antifungal agents. All the derivatives belonging to the series I, II and III showed antimicrobial activity comparable to the standard. Compounds I(f) and II(c) proved to be the most effective compounds. Quantitative structure-activity relationship
-
[EN] COSMETIC USE OF PIPERIDINE DERIVATIVES<br/>[FR] UTILISATION COSMETIQUE DE DERIVES DE LA PIPERIDINE申请人:OREAL公开号:WO2005123678A1公开(公告)日:2005-12-29The present invention relates to the cosmetic use, as an agent for combating wrinkles, especially expression wrinkles, and/or for decontracting the skin and/or relaxing the features, of at least one piperidine derivative chosen from the compounds of formula (I) and the salts and optical isomers thereof. The invention also relates to novel piperidine der vatives, and also to cosmetic compositions containing them.
表征谱图
-
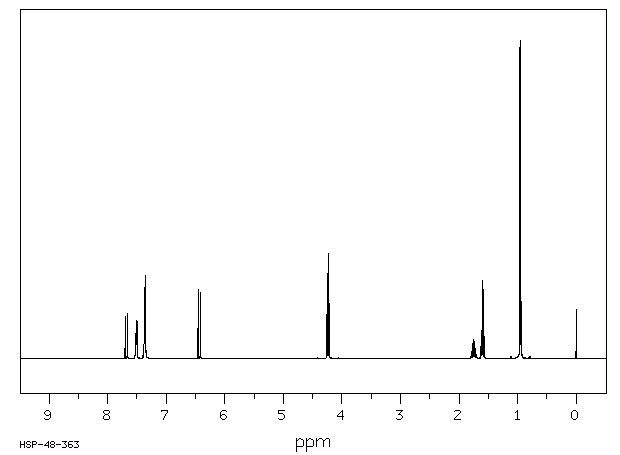
氢谱1HNMR
-
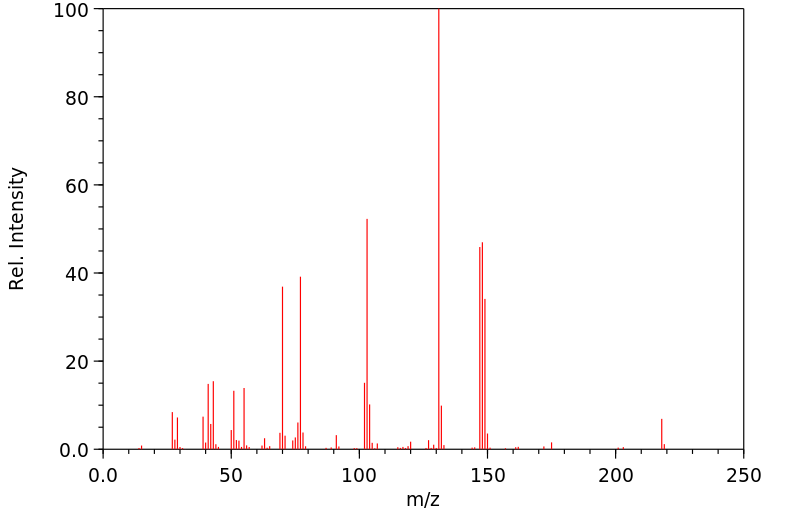
质谱MS
-
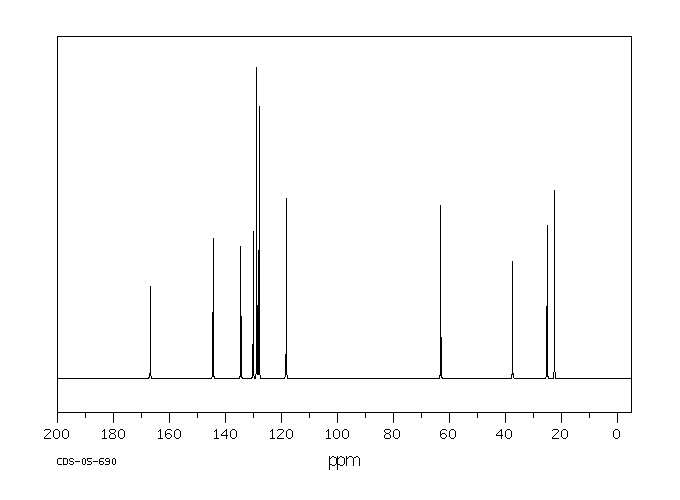
碳谱13CNMR
-
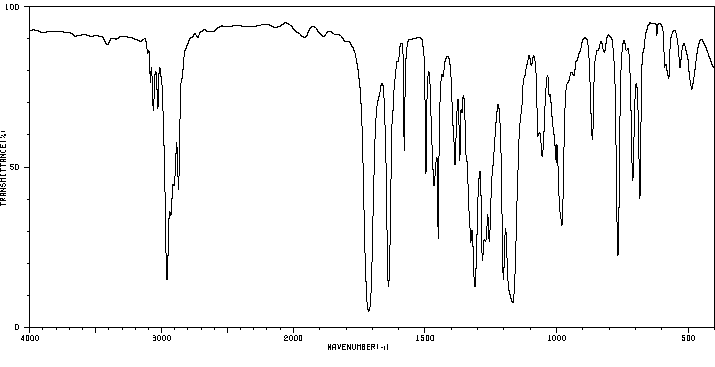
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30