二丙基丙二酸 | 1636-27-7
物质功能分类
中文名称
二丙基丙二酸
中文别名
二正丙基丙二酸;二-正丙基丙二酸
英文名称
di-n-propylmalonic acid
英文别名
dipropylmalonic acid;Dipropyl-malonsaeure;Di-n-propyl-malonsaeure;2,2-dipropylmalonic acid;2,2-dipropylpropanedioic acid
CAS
1636-27-7
化学式
C9H16O4
mdl
MFCD00051476
分子量
188.224
InChiKey
DIRSQLKNZQKDBK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:154-156°C
-
沸点:345.8±25.0 °C(Predicted)
-
密度:1.131±0.06 g/cm3(Predicted)
-
LogP:1.59
-
稳定性/保质期:
常温常压下稳定,避免氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:13
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.777
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险类别码:R36/37/38
-
海关编码:2917190090
-
安全说明:S26,S36
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
Dipropylmalonic Acid Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dipropylmalonic Acid
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dipropylmalonic Acid
Percent: >99.0%(T)
CAS Number: 1636-27-7
Synonyms: Heptane-4,4-dicarboxylic Acid
C9H16O4
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Dipropylmalonic Acid
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
Melting point/freezing point:158°C
Dipropylmalonic Acid
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Dipropylmalonic Acid
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dipropylmalonic Acid
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dipropylmalonic Acid
Percent: >99.0%(T)
CAS Number: 1636-27-7
Synonyms: Heptane-4,4-dicarboxylic Acid
C9H16O4
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Dipropylmalonic Acid
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
Melting point/freezing point:158°C
Dipropylmalonic Acid
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Dipropylmalonic Acid
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二甲基二丙基丙二酸酯 dimethyl (di-1-propyl)malonate 16644-05-6 C11H20O4 216.277 二丙基丙二酸二乙酯 2,2-dipropyl-malonic acid diethyl ester 6065-63-0 C13H24O4 244.331
反应信息
-
作为反应物:参考文献:名称:Fischer,E.; Dilthey, Chemische Berichte, 1902, vol. 35, p. 855摘要:DOI:
-
作为产物:描述:参考文献:名称:格氏试剂制备方法与应用摘要:本发明涉及一种格氏试剂的制备方法与应用,所述格氏试剂的结构式为:其以结构式为的卤代物为原料,用镁在有机溶剂中处理得到,其中,X为卤原子,从Cl、Br、I中选取,R2是含有2‑9个碳原子的烷基或芳基,所述格氏试剂主要应用于地莫匹醇及其盐酸盐的合成工艺,用以引入烷基侧链。本发明可以使地莫匹醇及其盐酸盐的整个合成过程安全而高效,其中每步反应操作简单、安全,且反应收率高,因此可以实现工业化生产。公开号:CN112047964A
文献信息
-
METHOD FOR PREPARING QUATERNARY PHOSPHONIUM SALTS申请人:Microvast Power Systems Co.,Ltd.公开号:US20150166587A1公开(公告)日:2015-06-18A two-step pathway for preparing high pure quaternary phosphonium salts is disclosed. In the first step, hydrogen phosphide (PH 3 ) or a higher phophine reacts with a protonic compound to produce a phosphonium salt, which then reacts with a carbonic acid diester to produce a quaternary phosphonium salt in the second step. On one hand, hydrogen phosphide (PH 3 ) and higher phophines, including primary phosphines, secondary phosphines, and tertiary phosphines, after neutralization with protonic compound, become sufficiently reactive and can be alkylated by carbonic acid diester to form quaternary phosphonium cations. On the other hand, as an anion-exchange procedure is completely avoided, the process not only gives quaternary phosphonium salts of high purity, but also gives people freedom to design the cation and the anion of a quaternary phosphonium salt synchronously by choosing a preferred phosphine and a protonic compound that can supply a desired anion.
-
COALESCING AGENT DERIVED FROM DIOXOLANE DERIVATIVES申请人:PTT GLOBAL CHEMICAL PUBLIC COMPANY LIMITED公开号:US20190194157A1公开(公告)日:2019-06-27The present invention relates to a coalescing agent as represented in structure (I); wherein; n is integer from 1 to 8; R 1 and R 2 independently represent group selected from hydrogen atom, alkyl, alkenyl, alkynyl, phenyl, benzyl groups, or optionally cyclic hydrocarbon containing heteroatom; and Y represents group selected from alkyl, alkenyl, alkynyl, phenyl, benzyl groups, or cyclic hydrocarbon containing heteroatom. The said coalescing agent can be used in coating application with efficacy to provide smooth consistent film with chemical and scratch resistant and has no pungent odour, wherein the preparation method of this compound is simplify and employs less harmful chemicals.
-
[EN] CROSSLINKABLE HOLE TRANSPORT MOLECULE HAVING FOUR RADICAL POLYMERIZABLE GROUPS AND METHOD TO MAKE THE SAME<br/>[FR] MOLÉCULE RÉTICULABLE DE TRANSPORT DE TROUS PRÉSENTANT QUATRE GROUPES POLYMÉRISABLES PAR VOIE RADICALAIRE ET PROCÉDÉ POUR SA PRÉPARATION申请人:LEXMARK INT INC公开号:WO2017117124A1公开(公告)日:2017-07-06A crosslinkable hole transport molecule having four radical polymerizable groups to be used in a photoconductor to provide a medium for hole transport upon exposure to light and method to make the same is provided. Photoconductors using this hole transport molecule have improved mechanical wear resistance and excellent electrical properties. The crosslinkable hole transport molecule containing four radical polymerizable functional groups has the general structure exemplified below:提供一种可交联的孔传输分子,具有四个自由基可聚合基团,用于在光照作用下提供孔传输介质的光导体,并提供制备方法。使用这种孔传输分子的光导体具有改善的机械耐磨性和优异的电学特性。含有四个自由基可聚合功能基团的可交联孔传输分子具有以下示例的一般结构:
-
Novel antibacterial agents申请人:Christensen G. Burton公开号:US20070134729A1公开(公告)日:2007-06-14This invention relates to novel multibinding compounds (agents) that are antibacterial agents. The multibinding compounds of the invention comprise from 2-10 ligands covalently connected by a linker or linkers, wherein each of said ligands in their monovalent (i.e., unlinked) state have the ability to bind to a an enzyme involved in cell wall biosynthesis and metabolism, a precursor used in the synthesis of the bacterial cell wall and/or the bacterial cell surface thereby interfere with the synthesis and/or metabolism of the cell wall. In particular the multibinding compounds of the invention comprise from 2-10 ligands covalently connected by a linker or linkers, wherein each of said ligands has a ligand domain capable of binding to penicillin binding proteins, a transpeptidase enzyme, a substrate of a transpeptidase enzyme, a beta-lactamase enzyme, pencillinase enzyme, cephalosporinase enzyme, a transglycoslase enzyme, or a transglycosylase enzyme substrate; Preferably, the ligands are selected from the beta lactam or glycopeptide class of antibacterial agents.
-
THERAPEUTICAL USE OF TERNARY COMPLEXES OF VALPROIC ACID申请人:Sylla-Iy Arreta Veitia Maite公开号:US20120142658A1公开(公告)日:2012-06-07A method of treating a human subject having a condition responsive to valproic acid therapy, includes administering to the subject an effective amount of a metal-based ternary complex of valproic acid with nitrogen donor ligands, in particular with diimines or diamines.
表征谱图
-
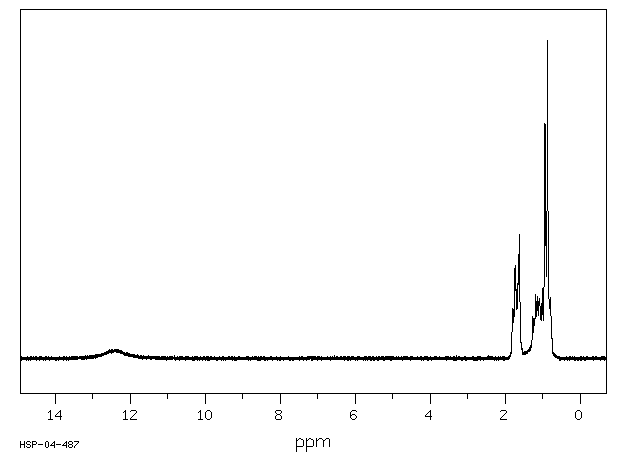
氢谱1HNMR
-
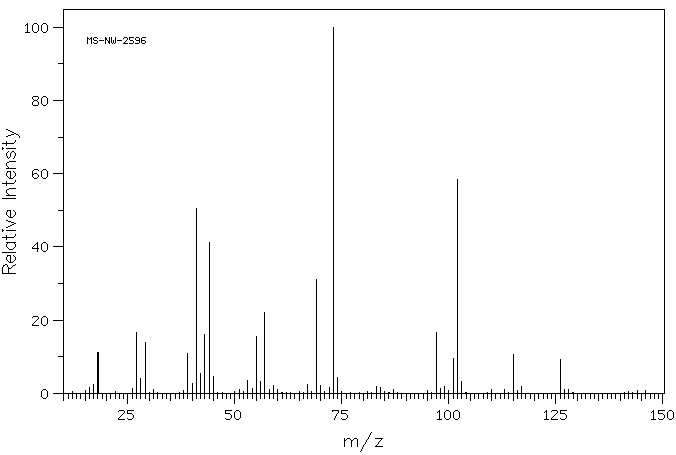
质谱MS
-
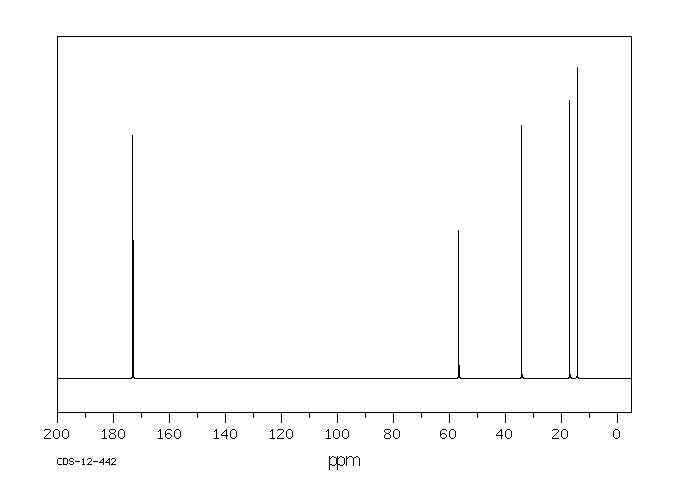
碳谱13CNMR
-
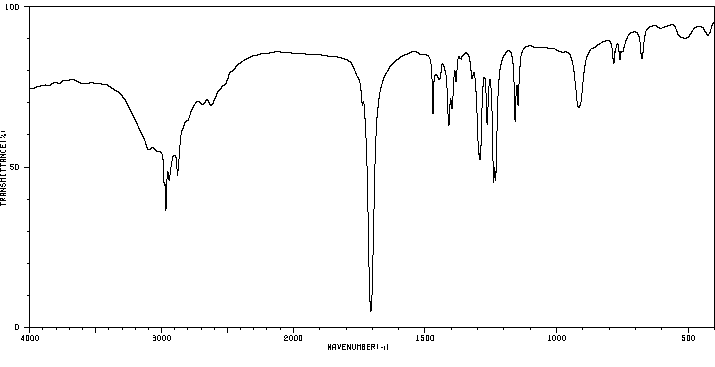
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯