4-(2-氯乙氧基)苯甲醛 | 54373-15-8
中文名称
4-(2-氯乙氧基)苯甲醛
中文别名
依曲替酯;苯壬四烯酯;N-(2-溴乙氧基)苯甲醛
英文名称
4-(2-chloroethoxy)benzaldehyde
英文别名
——
CAS
54373-15-8
化学式
C9H9ClO2
mdl
MFCD00043699
分子量
184.622
InChiKey
HBHHMVNKQWECIS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25-30°C
-
沸点:138-142 °C(Press: 2 Torr)
-
密度:1.2246 g/cm3(Temp: 25 °C)
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2913000090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:室温和干燥环境
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-(2-Chloroethoxy)benzaldehyde
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(2-Chloroethoxy)benzaldehyde
CAS number: 54373-15-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H9ClO2
Molecular weight: 184.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-(2-Chloroethoxy)benzaldehyde
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(2-Chloroethoxy)benzaldehyde
CAS number: 54373-15-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H9ClO2
Molecular weight: 184.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对羟基苯甲醛 4-hydroxy-benzaldehyde 123-08-0 C7H6O2 122.123 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4,4-乙烷二氧基二苯甲醛 1,2-bis(4-formylphenoxy)ethane 34074-28-7 C16H14O4 270.285 4-[2-(二甲胺基)乙氧基]苯甲醛 4-(2-dimethylaminoethoxy)benzaldehyde 15182-92-0 C11H15NO2 193.246 4-(2-(二乙基氨基)乙氧基)-苯甲醛 4-[2-(diethylamino)ethoxy]benzaldehyde 15182-94-2 C13H19NO2 221.299 4-(2-吗啉乙氧基)苯甲醛 4-(2-morpholin-4-yl-ethoxy)-benzaldehyde 82625-45-4 C13H17NO3 235.283 —— 4-(2-(pyrrolidin-1-yl)ethoxy)benzaldehyde 26116-47-2 C13H17NO2 219.283 4-[2-(4-甲基哌嗪-1-基)-乙氧基]-苯甲醛 4-(2-(4-methylpiperazin-1-yl)ethoxy)benzaldehyde 100875-69-2 C14H20N2O2 248.325 4-(2-哌啶-1-乙氧基)苯甲醛 4-(2-(piperidin-1-yl)ethoxy)benzaldehyde 26815-04-3 C14H19NO2 233.31 4-(2-氯乙氧基)苯酚 4-(2-chloroethoxy)phenol 100238-55-9 C8H9ClO2 172.611 4-[2-(1H-咪唑-1-基)乙氧基]苯甲醛 1盐酸盐 4-[2-(1H-imidazole-1-yl)ethoxy]benzaldehyde 205371-43-3 C12H12N2O2 216.239 —— (E)-3-(4-(2-chloroethoxy)phenyl)-1-phenylprop-2-en-1-one 1258631-46-7 C17H15ClO2 286.758
反应信息
-
作为反应物:描述:4-(2-氯乙氧基)苯甲醛 在 palladium on activated charcoal 哌啶 、 氢气 、 potassium carbonate 、 溶剂黄146 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 甲醇 、 N,N-二甲基甲酰胺 、 甲苯 为溶剂, 反应 3.0h, 生成 [[4-[2-(6-苯甲酰基-2-氧代-3(2H)-苯并噻唑基)乙氧基]苯基]甲基]-1,3-propanedioicaciddimethylester参考文献:名称:Novel 1,3-dicarbonyl compounds having 2(3H)-benzazolonic heterocycles as PPARγ agonists摘要:A series of 1,3-dicarbonyl compounds having 2(3H)-benzazolonic heterocycles has been synthesized and tested for PPAR gamma agonist activity. SAR were developed and revealed that 6-acyl-2(3H)-benzothiazolone derivatives with 1,3-dicarbonyl group were the most potent. IP administration of compound 22 exhibited comparable levels of glucose and triglyceride correction to PO administration of rosiglitazone in the oblob mouse studies. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2006.07.029
-
作为产物:描述:参考文献:名称:SUBSTITUTED 1-AMINOPHTHALAZINE DERIVATIVES, PREPARATION THEREOF AND THERAPEUTIC APPLICATION THEREOF摘要:这项发明涉及一般式(I)的1-氨基邻苯二酮衍生物:其中A、B、L、R、R1、R2、R3、R4、R5和R7如本文所定义。该发明还涉及所述化合物的制备及其治疗用途。公开号:US20090124624A1
文献信息
-
Effect of Oxime Ether Incorporation in Acyl Indole Derivatives on PPAR Subtype Selectivity作者:Morgan Le Naour、Veronique Leclerc、Amaury Farce、Daniel-Henri Caignard、Nathalie Hennuyer、Bart Staels、Valérie Audinot-Bouchez、Jean-Albert Boutin、Michel Lonchampt、Catherine Dacquet、Alain Ktorza、Pascal Berthelot、Nicolas LebegueDOI:10.1002/cmdc.201200316日期:2012.12balanced activity for both subtypes. Herein we report the discovery, synthesis, and optimization of a new series of α‐ethoxyphenylpropionic acid bearing 5‐ or 6‐substituted indoles. The incorporation of oxime ethers on the carbonyl portion of the benzoyl group can bring the PPARα/γ potency ratio equal to or slightly greater than one, as is the case for compounds 20 c and 21 a. Compound 20 c shows high efficacy同时激活过氧化物酶体增殖物激活受体(PPAR)亚型α和γ的化合物具有在单个药物活性分子中有效治疗血脂异常和2型糖尿病(T2D)的潜力。选择性添加PPARα活性有望克服选择性PPARγ激动剂经常观察到的副作用,例如水肿和体重增加,从而导致两种亚型的双重PPARα/γ激动剂均具有平衡的活性。本文中,我们报告发现,合成和优化了一系列新的带有5或6个取代的吲哚的α-乙氧基苯基丙酸。在苯甲酰基的羰基部分上掺入肟醚可以使PPARα/γ效力比等于或略大于1,就像化合物20c和21a的情况一样。化合物20c的表现出高效力在OB / OB T2D和血脂异常,类似于罗格列酮和替格列扎的小鼠模型,但与体重增加一个显著增加。与此相反,化合物21,作为双PPARα/γ活化剂小于有力20c中,显示了一个有趣的药理学特性,因为它引起在相对于体重的参考化合物的降低。
-
Asymmetric Synthesis of Chiral 1, <scp>3‐Disubstituted</scp> Allylsilanes <i>via</i> Copper(I)‐Catalyzed 1, <scp>4‐Conjugate</scp> Silylation of α, <scp>β‐Unsaturated</scp> Sulfones and Subsequent <scp>Julia‐Kocienski</scp> Olefination作者:Xian‐Liang Wang、Xing‐Hao Yin、Jun‐Zhao Xiao、Xue‐Shun Jia、Liang YinDOI:10.1002/cjoc.202100101日期:2021.7array of chiral allylsilanes in moderate yields. More interestingly, a one-pot asymmetric synthesis with high synthetic efficiency is successfully realized. Utility of the prepared chiral 1,3-disubsituted allylsilanes is demonstrated in the asymmetric allylation of both aldehyde and aldimine. Finally, an interesting “match and mismatch” phenomenon is observed in the asymmetric allylation of chiral aldehydes
-
Multicomponent formation route to a new class of oxygen-based 1,3-dipoles and the modular synthesis of furans作者:Huseyin Erguven、Cuihan Zhou、Bruce A. ArndtsenDOI:10.1039/d1sc04088j日期:——generated by the multicomponent reaction of aldehydes, acid chlorides and the phosphonite PhP(catechyl). These 1,3-dipoles are formally cyclic tautomers of simple Wittig-type ylides, where the angle strain and moderate nucleophilicity in the catechyl-phosphonite favor their cyclization and also direct 1,3-dipolar cycloaddition to afford single regioisomers of substituted products. Coupling the generation
-
New heterocyclic oxime compounds申请人:Leclerc Veronique公开号:US20050026973A1公开(公告)日:2005-02-03Compounds of formula (I): wherein: X represents oxygen or sulphur or a group CH 2 or R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are as defined in the description, A represents an alkylene chain as defined in the description, B represents alkyl or alkenyl substituted by a group or R 9 , or B represents a group or R 9 , D represents a benzene, pyridine, pyrazine, pyrimidine or pyridazine nucleus. Medicaments
-
[EN] PROTEOLYSIS TARGETING CHIMERA (PROTACS) AS DEGRADERS OF SMARCA2 AND/OR SMARCA4<br/>[FR] CHIMÈRES CIBLANT LA PROTÉOLYSE (PROTAC) SERVANT D'AGENTS DE DÉGRADATION DE SMARCA2 ET/OU SMARCA4申请人:BOEHRINGER INGELHEIM INT公开号:WO2020078933A1公开(公告)日:2020-04-23The present invention encompasses compounds of formula (I) wherein the groups R1,A, G, LK and t have the meanings given in the claims and specification, their use as degraders of SMARCA2 and/or SMARCA4, pharmaceutical compositions which contain compounds of this kind and their use as medicaments/medical uses, especially as agents for treatment and/or prevention of oncological diseases.本发明涵盖了式(I)中R1、A、G、LK和t所表示的基团,其用作SMARCA2和/或SMARCA4的降解剂,含有此类化合物的药物组合物以及它们作为药物/医用的用途,特别是作为治疗和/或预防肿瘤疾病的药剂/医疗用途的代理。
表征谱图
-
氢谱1HNMR
-
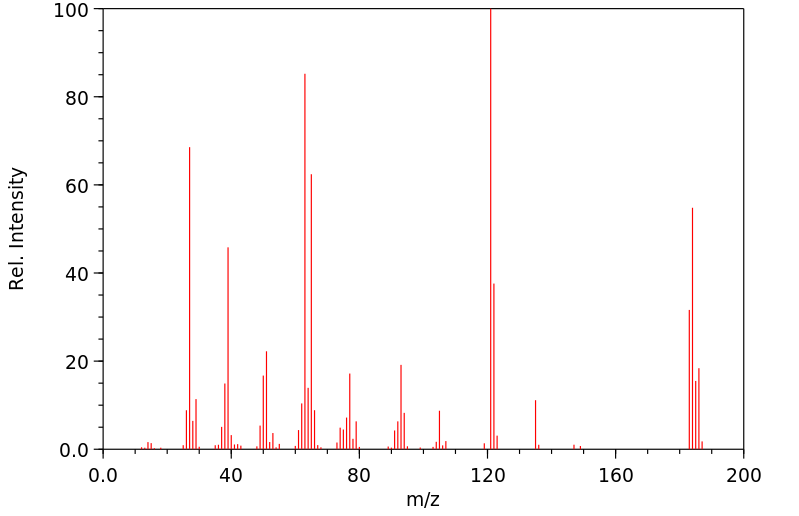
质谱MS
-
碳谱13CNMR
-
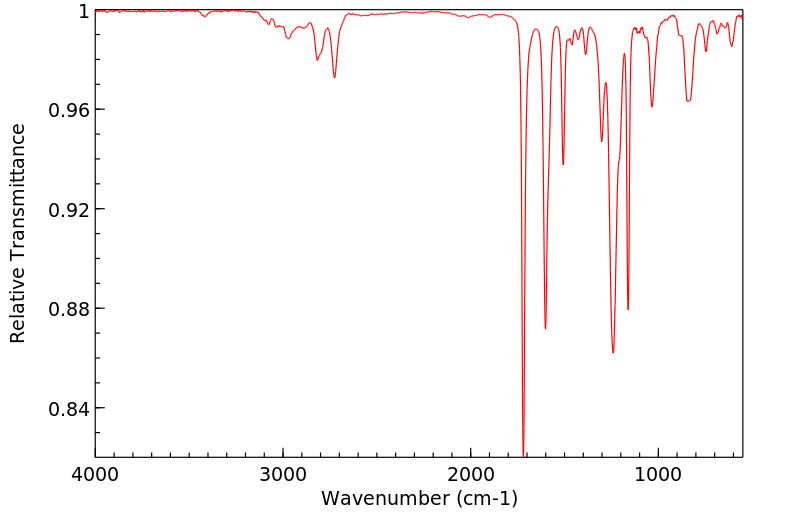
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯