5-羟基-4-辛酮 | 496-77-5
中文名称
5-羟基-4-辛酮
中文别名
5-羥-4-辛酮;丁偶姻
英文名称
butyroin
英文别名
5-hydroxyoctan-4-one;5-hydroxy-4-octanone
CAS
496-77-5
化学式
C8H16O2
mdl
——
分子量
144.214
InChiKey
BVEYJWQCMOVMAR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-10°C
-
沸点:80-82 °C10 mm Hg(lit.)
-
密度:0.916 g/mL at 25 °C(lit.)
-
闪点:175 °F
-
LogP:1.69
-
物理描述:yellowish liquid
-
溶解度:almost insoluble in water; soluble in alcohol
-
折光率:1.426-1.432
-
保留指数:1442.5
-
稳定性/保质期:
- 按规格使用和贮存,不会发生分解,避免与氧化物接触。
- 存在于主流烟气中。
- 天然品存在于可可等中。
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.88
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
WGK Germany:3
-
海关编码:2914400090
-
危险性防范说明:P210,P280,P370+P378,P403+P235,P501
-
危险性描述:H227,H302
-
储存条件:密封保存,置于通风、干燥的环境中。
SDS
丁偶姻 修改号码:5
模块 1. 化学品
产品名称: Butyroin
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 丁偶姻
百分比: >96.0%(GC)
CAS编码: 496-77-5
俗名: 5-Hydroxy-4-octanone
分子式: C8H16O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
丁偶姻 修改号码:5
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 浅黄色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 96 °C/2.7kPa
闪点: 77°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.92
丁偶姻 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
丁偶姻 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Butyroin
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 丁偶姻
百分比: >96.0%(GC)
CAS编码: 496-77-5
俗名: 5-Hydroxy-4-octanone
分子式: C8H16O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
丁偶姻 修改号码:5
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 浅黄色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 96 °C/2.7kPa
闪点: 77°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.92
丁偶姻 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
丁偶姻 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (R)-5-hydroxyoctan-4-one —— C8H16O2 144.214
反应信息
-
作为反应物:描述:参考文献:名称:1, 2-二醇,α-酮和 1, 2-二酮与次氯酸钠水溶液的氧化裂解摘要:1,2-二醇的氧化裂解在有机合成中有多种应用。虽然过氧化氢钨酸盐或磷酸盐等氧化剂,'N-碘代琥珀酰亚胺?NBS-四(正丁基)碘化铵?Jones 试剂”,据报道,最常用于裂解 12-二醇的试剂是高碘酸和四乙酸铅!高碘酸主要用于降解水溶性二醇,而四乙酸铅主要用于不溶于水的二醇. 使用这些和其他试剂切割 1,2-二醇存在局限性和困难,例如成本因素、反应条件和麻烦的后处理。'* 寻找一种实用且有效的试剂来切割各种 1,2 的 CC 键-二醇、α-酮醇和 1,2-二酮一直是我们关注的焦点。我们已经描述了次氯酸钠水溶液的不同应用?-l0 本文报道了一种简单、方便且廉价的方法,用于用钠水溶液氧化裂解水溶性和水不溶性 1,2-二醇、a-酮醇和 1,2-二酮次氯酸盐的乙腈溶液,室温。结果在表I中描述。当底物与 NaOCl(2a-2j) 的摩尔比为 1:20 时,各种 1,2-二芳基二烷基-12-二醇 (la-11)DOI:10.1080/00304940709356010
-
作为产物:描述:参考文献:名称:Ruehlmann,K., Synthesis, 1971, p. 236 - 253摘要:DOI:
-
作为试剂:参考文献:名称:Process for producing .alpha.-hydroxyketones摘要:一种制备式(I)所表示的α-羟基酮的方法:其中R1代表取代或未取代的烷基、取代或未取代的芳基烷基或烷氧羰基;R2和R3分别代表氢原子、取代或未取代的烷基或取代或未取代的芳基烷基,但不能同时代表氢原子;或者R1和R2的一对、R1和R3的一对或R2和R3的一对被取为一个环;并且R1和R2的一对和R2和R3的一对可以同时形成一个环。该方法包括在钌化合物和水的存在下,使用氧化剂反应式(II)所表示的化合物:其中R1、R2和R3如上所定义。以良好的选择性和高收率制备出用作生理活性物质的α-羟基酮。公开号:US05210315A1
文献信息
-
Prodrugs of GABA analogs, compositions and uses thereof申请人:Gallop A. Mark公开号:US20060229361A1公开(公告)日:2006-10-12The present invention provides prodrugs of GABA analogs, pharmaceutical compositions of prodrugs of GABA analogs and methods for making prodrugs of GABA analogs. The present invention also provides methods for using prodrugs of GABA analogs and methods for using pharmaceutical compositions of prodrugs of GABA analogs for treating or preventing common diseases and/or disorders.
-
Tandem Hydroformylation/Acyloin Reaction - The Synergy of Metal Catalysis and Organocatalysis Yielding Acyloins Directly from Olefins作者:Karoline A. Ostrowski、Thiemo A. Faßbach、Andreas J. VorholtDOI:10.1002/adsc.201401031日期:2015.5.4A novel, atom efficient, orthogonal tandem catalysis was developed yielding acyloin products (α‐hydroxy ketones) directly from olefins under hydroformylation conditions. The combination of a metal‐catalysed hydroformylation and an organocatalysed acyloin reaction provides three atom efficient CC bond formations to linear, multifunctional molecules via linkage of the intermediate n‐aldehydes. Additionally
-
Convenient Preparation of 'High-Surface Sodium' in Liquid Ammonia: use in the Acyloin Reaction作者:Mieczyslaw Makosza、Karol GrelaDOI:10.1055/s-1997-763日期:1997.3'Sodium on solid support' (5-20 wt.% of NaCl, glass powder, poly(ethylene) and poly(propylene)) can be conveniently prepared via low-temperature (-33°C) deposition of sodium from its solution in liquid ammonia. Use of this reagent in the acyloin reaction of carboxylic esters gave the corresponding products in good yields.
-
Direct Catalytic Transformation of Olefins into α-Hydroxy Ketones with Hydrogen Peroxide Catalyzed by Peroxotungstophosphate作者:Yasuyuki Sakata、Yuji Katayama、Yasutaka IshiiDOI:10.1246/cl.1992.671日期:1992.4Aliphatic olefins were directly converted into α-hydroxy ketones with acidic aqueous hydrogen peroxide in the presence of catalytic amount of peroxotungstophosphate (PCWP) under the biphasic system using chloroform as a solvent. The acidic medium was necessary to open the resulting epoxide to vic-diol which was subsequently oxidized to α-hydroxy ketones.
-
NaBrO3/bmim[HSO4]: a versatile system for the selective oxidation of 1,2-diols, α-hydroxyketones, and alcohols作者:Jitender M. Khurana、Anshika Lumb、Ankita ChaudharyDOI:10.1007/s00706-016-1749-z日期:2017.2found to be an excellent oxidizing agent in aqueous medium. NaBrO3:bmim[HSO4] oxidized 1,2-diols, α-hydroxyketones, and alcohols to the corresponding carbonyl compounds in excellent yields. This method offers advantages such as low cost reagents, aqueous reaction conditions, moderate temperatures and short reaction times and hence environmentally benign reaction. Moreover, the ionic liquid bmim[HSO4] could
表征谱图
-
氢谱1HNMR
-
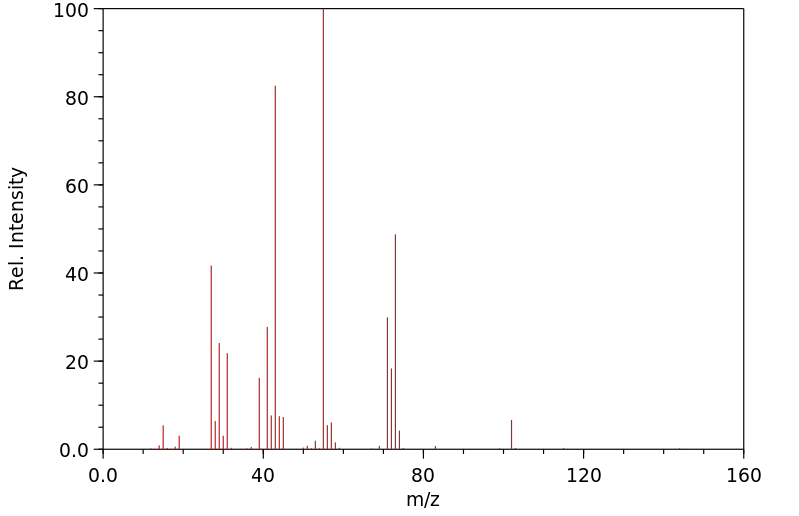
质谱MS
-
碳谱13CNMR
-
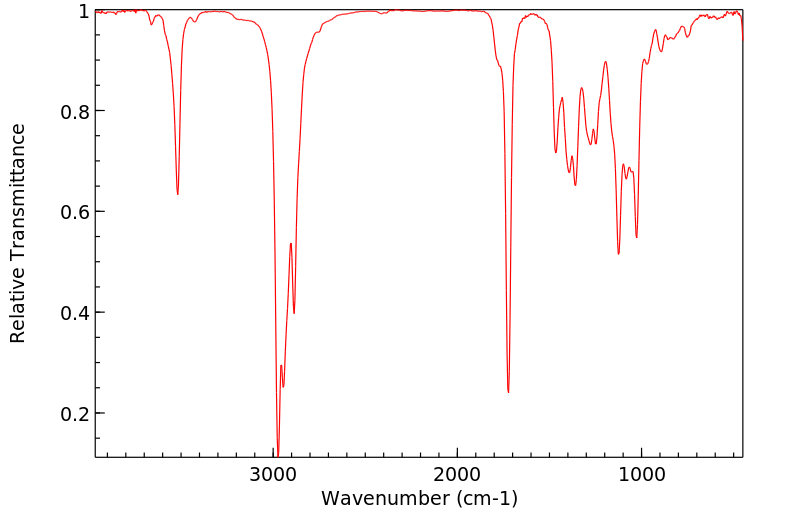
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷