17Alpha-羟基孕烯醇酮-17-乙酸酯 | 2381-45-5
物质功能分类
分子结构分类
中文名称
17Alpha-羟基孕烯醇酮-17-乙酸酯
中文别名
17Α-乙酰氧基孕烯醇酮;17α-羟基孕烯醇酮-17-乙酸酯;乙酸-17α-羟基孕烯醇酮-17-酯;17-乙酸-17α-
英文名称
pregn-5-ene-3β,17α-diol-20-one 17-acetate
英文别名
17α-Acetoxy-3β-hydroxypregn-5-en-20-one;17α-acetoxy-3β-hydroxy-5-pregnen-20-one;17-acetoxy-3β-hydroxy-pregn-5-en-20-one;17-Acetoxy-3β-hydroxy-pregn-5-en-20-on;3-hydroxy-20-oxo-5-pregnen-17α-yl acetate;17α-acetoxypregnenolone;3-Hydroxy-20-oxo-5-pregnen-17alpha-yl acetate;[(3S,8R,9S,10R,13S,14S,17R)-17-acetyl-3-hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl] acetate
CAS
2381-45-5
化学式
C23H34O4
mdl
——
分子量
374.521
InChiKey
KRBMZSYRIJAAMS-KIYFCUGLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
溶解度:可溶于二氯甲烷;乙酸乙酯
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:27
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:63.6
-
氢给体数:1
-
氢受体数:4
安全信息
-
安全说明:S24/25
SDS
| Name: | 17alpha-Acetoxypregnenolone Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2381-45-5 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2381-45-5 | 17alpha-acetoxypregnenolone | 100 | 219-180-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Dust may cause mechanical irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause digestive tract disturbances. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2381-45-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white to tan
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 230.00 - 232.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C23H34O4
Molecular Weight: 374.51
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2381-45-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
17alpha-acetoxypregnenolone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2381-45-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2381-45-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2381-45-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (3beta)-3-(甲酰氧基)-20-氧代孕甾-5-烯-17-基乙酸酯 17-acetoxy-3β-formyloxy-pregn-5-en-20-one 83984-86-5 C24H34O5 402.531 (17-乙酰基-3-乙酰氧基-10,13-二甲基-1,2,3,4,7,8,9,11,12,14,15,16-十二氢环戊烯并[a]菲-17-基)乙酸酯 3β,17α-diacetoxypregn-5-en-20-one 3517-38-2 C25H36O5 416.558 孕甾-3,5-二烯-3Β,17Α-二醇-20-酮-3,17-二醋酸酯 3,17α-diacetoxy-3,5-pregnadien-20-one 4954-07-8 C25H34O5 414.542 17Alpha-羟基黄体酮 17-hydroxyprogesterone 68-96-2 C21H30O3 330.467 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (17-乙酰基-3-乙酰氧基-10,13-二甲基-1,2,3,4,7,8,9,11,12,14,15,16-十二氢环戊烯并[a]菲-17-基)乙酸酯 3β,17α-diacetoxypregn-5-en-20-one 3517-38-2 C25H36O5 416.558 17Alpha-羟基妊娠烯醇酮 17-Hydroxypregnenolone 387-79-1 C21H32O3 332.483 —— 17α-acetoxy-6α-fluoropregn-4-ene-3,20-dione 336-78-7 C23H31FO4 390.495
反应信息
-
作为反应物:描述:参考文献:名称:15β-hydroxysteroids (Part V). Steroids of the human perinatal period: The synthesis of 3β, 15β, 17α-trihydroxy-5-pregnen-20-one from 15β, 17α-dihydroxy-4-pregnen-3,20-dione摘要:A simple three-step synthetic method is reported on the conversion of Delta(4)-3-ketosteroids to the corresponding 3 beta-hydroxy-Delta(5)-steroid analogues. 17 alpha-Hydroxy-4-pregnen-3,20-dione (10a) was used as a model to develop a method for the synthesis of 3 beta, 17 alpha-dihydroxy-5-pregnen-20-one (16). The major problem being the synthesis of 3,17 alpha-diacetoxy-3,5-pregnadien-20-one(14) was solved by acetylating using a mixture of acetic anhydride and perchloric acid. The conversion of 15 beta, 17 alpha-dihydroxy-4-pregnen-3,20-dione (8), product of Penicillium citrinum fermentation, to the desired 3 beta,15 beta 17 alpha-trihydroxy-5-pregen-20-one (1), is described using a modification of this method. Reaction of 8 with acetic anhydride and perchloric acid in ethyl acetate gave 3, 15 beta,17 alpha-triacetoxy-3,5-pregnadien-20-one (17) which on reduction with sodium borohydride gave 5-pregnen-3 beta,15 beta,17 alpha,20(S + R)-tetrols (18a and 18b); however, reduction of 17 with a mixture of sodium borohydride and potassium bicarbonate gave after basic hydrolysis with methanolic sodium hydroxide the desired product 3 beta, 15 beta,17 alpha-trihydroxy-5-pregnen-20-one (1) in good yield (54%).DOI:10.1016/0039-128x(95)00170-u
-
作为产物:描述:17Alpha-羟基黄体酮 在 sodium tetrahydroborate 、 高氯酸 作用下, 以 乙醇 、 乙酸乙酯 为溶剂, 反应 0.1h, 生成 17Alpha-羟基孕烯醇酮-17-乙酸酯参考文献:名称:15β-hydroxysteroids (Part V). Steroids of the human perinatal period: The synthesis of 3β, 15β, 17α-trihydroxy-5-pregnen-20-one from 15β, 17α-dihydroxy-4-pregnen-3,20-dione摘要:A simple three-step synthetic method is reported on the conversion of Delta(4)-3-ketosteroids to the corresponding 3 beta-hydroxy-Delta(5)-steroid analogues. 17 alpha-Hydroxy-4-pregnen-3,20-dione (10a) was used as a model to develop a method for the synthesis of 3 beta, 17 alpha-dihydroxy-5-pregnen-20-one (16). The major problem being the synthesis of 3,17 alpha-diacetoxy-3,5-pregnadien-20-one(14) was solved by acetylating using a mixture of acetic anhydride and perchloric acid. The conversion of 15 beta, 17 alpha-dihydroxy-4-pregnen-3,20-dione (8), product of Penicillium citrinum fermentation, to the desired 3 beta,15 beta 17 alpha-trihydroxy-5-pregen-20-one (1), is described using a modification of this method. Reaction of 8 with acetic anhydride and perchloric acid in ethyl acetate gave 3, 15 beta,17 alpha-triacetoxy-3,5-pregnadien-20-one (17) which on reduction with sodium borohydride gave 5-pregnen-3 beta,15 beta,17 alpha,20(S + R)-tetrols (18a and 18b); however, reduction of 17 with a mixture of sodium borohydride and potassium bicarbonate gave after basic hydrolysis with methanolic sodium hydroxide the desired product 3 beta, 15 beta,17 alpha-trihydroxy-5-pregnen-20-one (1) in good yield (54%).DOI:10.1016/0039-128x(95)00170-u
文献信息
-
Enzymatic Production or Chemical Synthesis and Uses for 5,7-Dienes and UVB Conversion Products Thereof申请人:Slominski Andrej公开号:US20120258938A1公开(公告)日:2012-10-11Provided herein are steroidal compounds that are androsta-5,7-dienes or pregna-5,7-dienes and ultraviolet B (UVB) conversion products thereof and cholecalciferol derivatives hydroxylated at one or more of C1, C17, C20, C23, C24, C25, and C26 which includes pharmaceutical, cosmeceutical or nutraceutical compositions of the steroidal compounds as shown in Tables 1A, 2A and 3. Also provided is a method for producing hydroxylated metabolites of cholecalciferol via CYP11A1, CYP24, CYP27A1, or CYP27B1 enzyme systems where the hydroxylase has an activity to hydroxylate position C1 or C20 or other position of the sidechain of a secosteroid or its 5,7-dieneal precursor and the hydroxylated metabolites so produced. Methods are provided for inhibiting proliferation of either a normally or abnormally proliferating cell, for modifying immune activity, or for treating a condition associated with the proliferating or quiescent cell or immune cells by contacting the cell with or administering any of the compounds described herein.本文提供的是雄甾-5,7-二烯或孕甾-5,7-二烯及其紫外线B(UVB)转化产物和羟化在C1、C17、C20、C23、C24、C25和C26上的胆钙醇衍生物,其中包括表1A、2A和3中所示的雄甾类化合物的制药、化妆品或营养品组合物。同时,本文还提供了一种通过CYP11A1、CYP24、CYP27A1或CYP27B1酶系统产生羟化代谢产物的方法,其中羟化酶具有羟化分裂素或其5,7-二烯前体的侧链的C1或C20或其他位置的活性,以及由此产生的羟化代谢产物。本文还提供了一种通过接触或给予本文所述的任何化合物来抑制正常或异常增殖细胞的增殖,修饰免疫活性或治疗与增殖或静止细胞或免疫细胞相关的疾病的方法。
-
Enzymatic production or chemical synthesis and uses for 5,7-dienes and UVB conversion products thereof申请人:Slominski Andrzej公开号:US20110118228A1公开(公告)日:2011-05-19Provided herein are steroidal compounds that are androsta-5,7-dienes or a pregna-5,7-dienes and ultraviolet B (UVB) conversion products thereof which includes pharmaceutical compositions of the steroidal compounds as shown in Tables 1 and 2. Also provided is a method for producing hydroxylated metabolites of cholecalciferol or ergocalciferol via the P450scc (CYP11A1) or CYP27B1 enzyme systems where the hydroxylase has an activity to hydroxylate position C20 of a secosteroid or its 5,7-dieneal precursor and the hydroxylated metabolites so produced. In addition, a method for inhibiting proliferation of either a normally or abnormally proliferating cell by contacting the cell with any of the compounds described herein. A related method is provided of treating a condition associated with the proliferating cell such as a cancer, a skin disorder, a defect in cell differentiation, cosmetic, prophylaxsis, or healthy cell maintenance.
-
DECOLORIZABLE INK AND PRINTER申请人:——公开号:US20010008164A1公开(公告)日:2001-07-19A decoloring apparatus for an image forming material comprising means for bringing a solvent into contact with an image forming material formed on a paper sheet and containing a color former, a developer and a decolorizer, and means for removing a residual solvent from the paper sheet.一种用于图像形成材料的脱色装置,包括使溶剂与形成在纸页上的图像形成材料接触的装置,其中包含显色剂、显影剂和脱色剂,以及从纸页上去除残留溶剂的装置。
-
Method of forming subtraction images申请人:Arakawa Satoshi公开号:US20050195943A1公开(公告)日:2005-09-08A radiation image signal is acquired from a radiation image of an object before being injected with a contrast medium, and a radiation image signal is acquired from a radiation image of the same object after being injected with the contrast medium. A subtraction process for subtracting image signal components of a plurality of the thus acquired radiation image signals, which image signal components represent corresponding pixels in the radiation images represented by the plurality of the radiation image signals, from one another is performed. A contrasted radiation image, in which a pattern of a specific structure of the object having been contrasted with the contrast medium in the radiation image has been extracted or enhanced, is thus formed. The contrast medium is a liposome, which contains a hydrophobic iodine compound as a film forming constituent.
-
Templeton, John F.; Kumar, V. P. Sashi; Kim, Ryungsoon S., Journal of the Chemical Society. Perkin transactions I, 1987, p. 1361 - 1368作者:Templeton, John F.、Kumar, V. P. Sashi、Kim, Ryungsoon S.、LaBella, Frank S.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
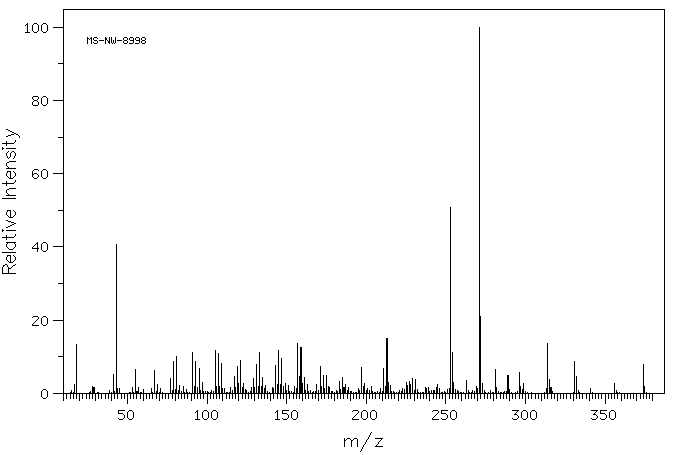
质谱MS
-
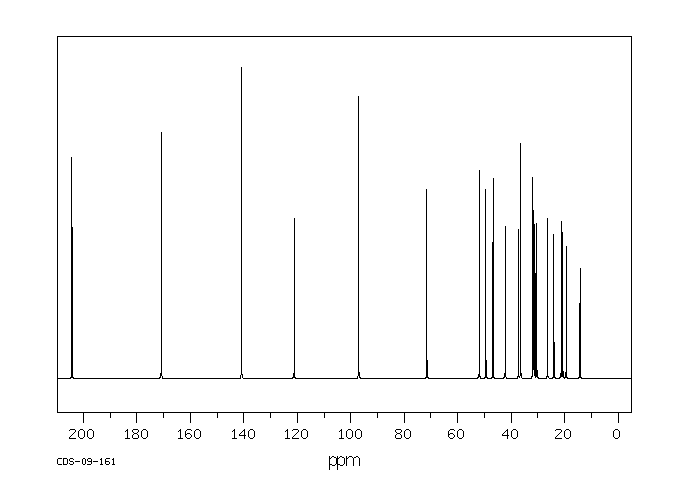
碳谱13CNMR
-
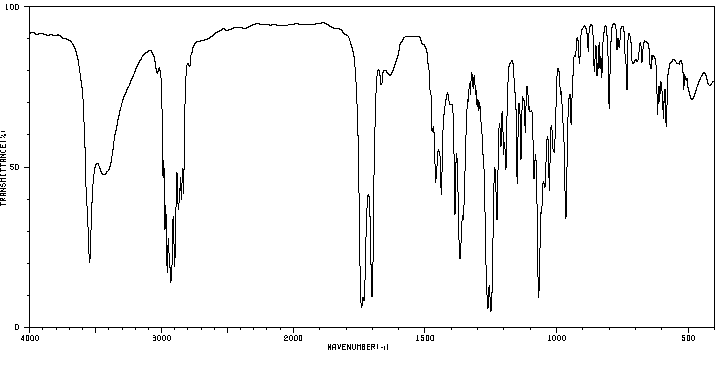
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B