camphor oxime | 37850-13-8
中文名称
——
中文别名
——
英文名称
camphor oxime
英文别名
(+)-Camphor oxime;(NE)-N-[(1R,4R)-1,7,7-trimethyl-2-bicyclo[2.2.1]heptanylidene]hydroxylamine
CAS
37850-13-8
化学式
C10H17NO
mdl
——
分子量
167.251
InChiKey
OVFDEGGJFJECAT-RZIOALPKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.9
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
反应信息
-
作为反应物:描述:camphor oxime 在 sodium hydride 作用下, 以 乙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 14.0h, 生成 (1R,4R)-1,7,7-Trimethyl-bicyclo[2.2.1]heptan-2-one O-(3-azepan-1-yl-2-hydroxy-propyl)-oxime参考文献:名称:O-[2-Hydroxy-3-(dialkylamino)propyl]ethers of (+)-1,7,7-trimethyl bicyclo[2.2.1]heptan-2-one oxime (camphor oxime) with analgesic and antiarrhythmic activities摘要:A novel series of O-[2-hydroxy-3-(dialkylamino)propyl]ethers of (+)-camphor oxime was prepared and tested for its cardiovascular, analgesic and anti-inflammatory properties. No significant anti-inflammatory and hypotensive activities were displayed by any of the compounds, whereas several of them are reasonably active as antiarrhythmic and analgesic agents. (C) 2000 Elsevier Science S.A. All rights reserved.DOI:10.1016/s0014-827x(00)00065-3
-
作为产物:描述:参考文献:名称:可见光光氧化还原催化苯乙烯与肟酯和 CO2 的双碳官能化:对氰基羧酸和 γ-酮酸的多组分反应摘要:已经实现了苯乙烯与肟酯和 CO 2的光氧化还原催化双碳官能化。值得注意的是,一系列四元、五元或六元环酮肟可以很好地以良好的产率提供各种 ε-、ζ- 和 η-氰基羧酸。此外,以无环酮肟酯为羰基自由基前驱体,还可以得到一系列γ-酮酸。它提供了对多种生物学上重要的氰基羧酸和 γ-酮酸的聚合访问。DOI:10.1021/acs.orglett.1c03938
文献信息
-
A modular, low footprint and scalable flow platform for the expedient α-aminohydroxylation of enolizable ketones作者:Victor-Emmanuel H. Kassin、Romain Morodo、Thomas Toupy、Isaline Jacquemin、Kristof Van Hecke、Raphaël Robiette、Jean-Christophe M. MonbaliuDOI:10.1039/d0gc04395h日期:——reactivity profile of α-chloronitroso derivatives is expressed to its fullest potential through the development of an integrated, modular and scalable continuous flow process for the electrophilic α-aminohydroxylation of various enolizable ketones. Flow conditions contribute to mitigating the high reactivity and toxicity of α-chloronitroso derivatives and provide an efficient, versatile and safe protocol for通过开发集成的,模块化的和可扩展的连续流工艺,用于各种可烯化酮的亲电性α-氨基羟基化,充分发挥了α-氯亚硝基衍生物的独特反应活性。流动条件有助于减轻α-氯亚硝基衍生物的高反应活性和毒性,并为占地面积最小的酮类α-氨基羟基化提供有效,通用和安全的方案。DFT计算了α-氨基羟基化过程的基本方面,并进一步支持了实验观察,因此导致了主要,次要和叔底物空前的基于α-氯亚硝基的α-氨基羟基化。α-氯亚硝基衍生物的碳主链的再循环为制备增值分子提供了高原子经济性。这项工作展示了α-氯亚硝基衍生物,是将羟胺的亲电合成子向亲核烯醇酸酯转移的经济有效的载体。根据完全连接的过程,几分钟内即可制备出具有代表性的一系列药物活性成分的前体和类似物,包括WHO必需品和短缺的药物(例如肾上腺素和氯胺酮)。该工艺具有顺序模块,具有不同的单元操作,包括化学转化和多次在线提取。该方法依赖于上游化学生成器,该化学生成器管理α-氯亚
-
Synthesis of esters of D, L-, D(+)-, and L(−)-camphor oximes: Structure-odor correlation作者:E. A. Dikusar、N. A. Zhukovskaya、O. G. VyglazovDOI:10.1134/s1070427206120147日期:2006.12Esters of D,L-, D(+)-, and L(-)-camphor oximes were synthesized, and the correlation between their structure and odor was examined.
-
N,N′-Dichloro bis(2,4,6-trichlorophenyl)urea (CC-2): an efficient reagent for the synthesis of α-chloro-nitroso compounds作者:A.K. Gupta、J. Acharya、D. Pardasani、D.K. DubeyDOI:10.1016/j.tetlet.2006.12.001日期:2007.1A simple, efficient, rapid, and mild method for the synthesis of alpha-chloro-nitroso compounds is described using bis(2,4,6-trichlorophenyl)urea (CC-2). (c) 2006 Elsevier Ltd. All rights reserved.
-
Schenone; Bruno; Ranise, Il Farmaco, 1994, vol. 49, # 9, p. 545 - 550作者:Schenone、Bruno、Ranise、Bondavalli、D'Amico、Vacca、Falciani、FilippelliDOI:——日期:——
-
Majeed, Nesreen N.; MacDougall, Gordon S.; Porte, Andrew L., Journal of the Chemical Society. Perkin transactions II, 1988, p. 1027 - 1034作者:Majeed, Nesreen N.、MacDougall, Gordon S.、Porte, Andrew L.、Sadler, Ian H.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
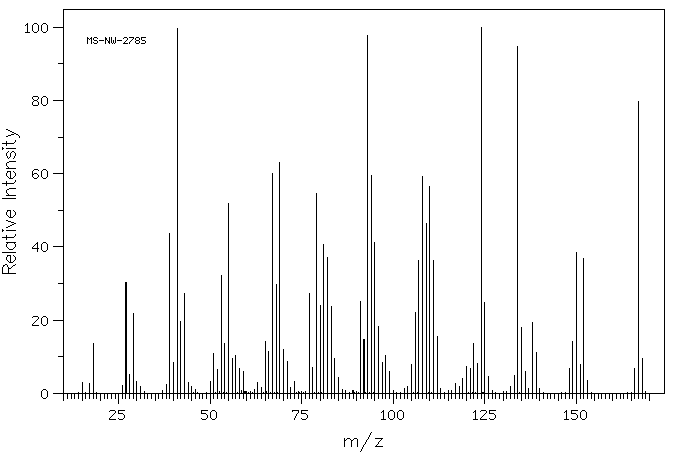
质谱MS
-
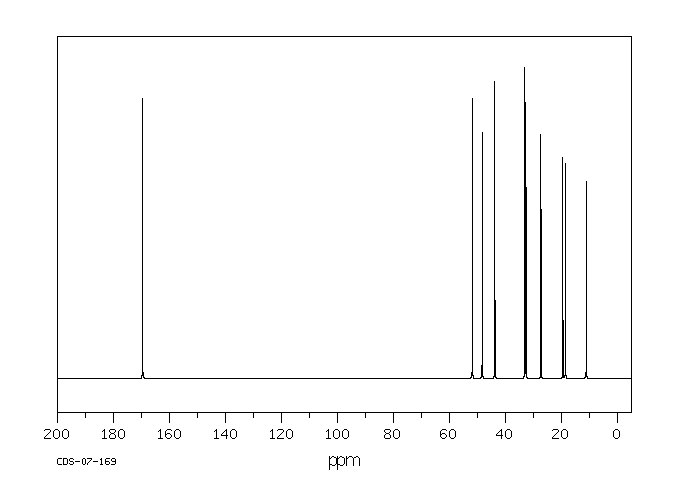
碳谱13CNMR
-
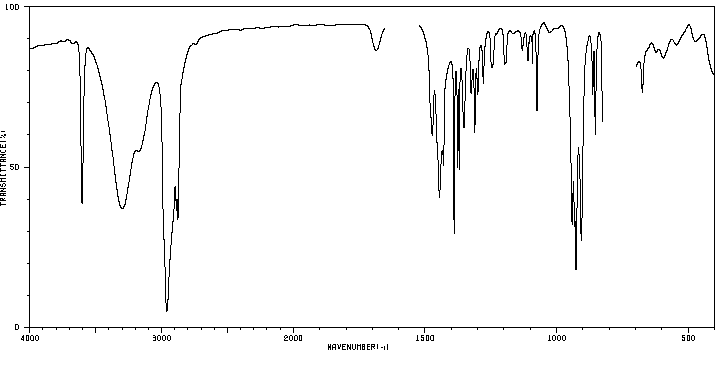
红外IR
-
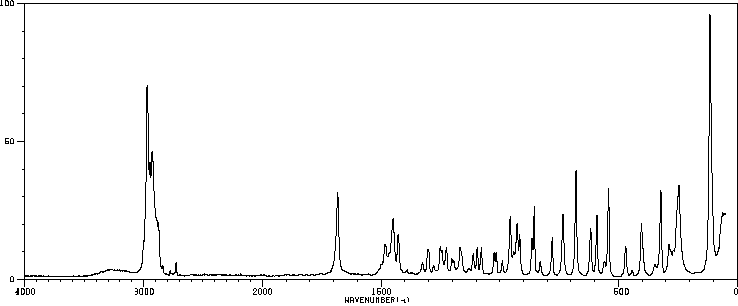
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸