5-phenethyldihydrofuran-2(3H)-one | 112606-95-8
中文名称
——
中文别名
——
英文名称
5-phenethyldihydrofuran-2(3H)-one
英文别名
2(3H)-Furanone, dihydro-5-(2-phenylethyl)-;5-(2-phenylethyl)oxolan-2-one
CAS
112606-95-8
化学式
C12H14O2
mdl
——
分子量
190.242
InChiKey
ONECXZFGYJSVIF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:173-175 °C(Press: 7 Torr)
-
密度:1.090±0.06 g/cm3(Predicted)
-
熔点:<25 °C
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.42
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:5-phenethyldihydrofuran-2(3H)-one 在 氯化亚砜 、 zinc(II) chloride 作用下, 反应 24.0h, 生成参考文献:名称:Catalytic Asymmetric γ-Alkylation of Carbonyl Compounds via Stereoconvergent Suzuki Cross-Couplings摘要:With the aid of a chiral nickel catalyst, enantioselective gamma- (and delta-) alkylations of carbonyl compounds can be achieved through the coupling of gamma-haloamides with alkylboranes. In addition to primary alkyl nucleophiles, for the first time for an asymmetric cross-coupling of an unactivated alkyl electrophile, an arylmetal, a boronate ester, and a secondary (cyclopropyl) alkylmetal compound are shown to couple with significant enantioselectivity. A mechanistic study indicates that cleavage of the carbon-halogen bond of the electrophile is irreversible under the conditions for asymmetric carbon-carbon bond formation.DOI:10.1021/ja2079515
-
作为产物:描述:1-phenylhex-5-yn-3-ol 在 甲烷磺酸 、 C21H12F9P*C8H18NO4S2(1-)*Au(1+) 、 间氯过氧苯甲酸 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 5.0h, 以86%的产率得到5-phenethyldihydrofuran-2(3H)-one参考文献:名称:金催化串联环化/氧化有效合成γ-内酯摘要:开发了一种新型的Au催化的炔丙基醇串联环异构化/氧化反应。利用这种策略可以很容易地获得各种γ-内酯。值得注意的是,该反应的机理与提出钌亚乙烯基中间体的相关Ru催化的反应明显不同。DOI:10.1021/ol302323a
文献信息
-
MnO<sub>2</sub>-promoted carboesterification of alkenes with anhydrides: a facile approach to γ-lactones作者:Lihuan Wu、Zhenming Zhang、Jianhua Liao、Jianxiao Li、Wanqing Wu、Huanfeng JiangDOI:10.1039/c5cc08867d日期:——
A new radical cyclization method for the formation of C(sp3)–C(sp3) and C–O bonds
via MnO2-promoted alkene carboesterification with anhydrides is developed.一种新的基于锰促进的烯烃碳酯化反应的C(sp3)-C(sp3)和C-O键的放射状环化方法被开发出来。 -
Gallium-catalyzed reductive lactonization of γ-keto acids with a hydrosilane作者:Norio Sakai、Shuhei Horikawa、Yohei OgiwaraDOI:10.1039/c6ra19286f日期:——Described herein is the GaCl3-catalyzed lactonization of γ-keto carboxylic acids in the presence of PhSiH3 leading to the direct preparation of γ-lactone derivatives. This reducing system showed a relatively wide functional group tolerance.
-
Indium-Catalyzed Direct Conversion of Lactones into Thiolactones and Selenolactones in the Presence of Elemental Sulfur and Selenium作者:Norio Sakai、Shuhei Horikawa、Yohei OgiwaraDOI:10.1055/s-0036-1591849日期:2018.2Abstract The direct conversion of lactones into thiolactones with elemental sulfur (S8) catalyzed by InCl3/PhSiH3 in a one-pot reaction is described. This catalytic system was successfully applied to the novel preparation of selenolactones from lactones and selenium. The direct conversion of lactones into thiolactones with elemental sulfur (S8) catalyzed by InCl3/PhSiH3 in a one-pot reaction is described
-
Efficient Synthesis of γ‐Lactones by Cobalt‐Catalyzed Carbonylative Ring Expansion of Oxetanes under Syngas Atmosphere作者:Yitian Tang、Chaoren Shen、Qiyi Yao、Xinxin Tian、Bo Wang、Kaiwu DongDOI:10.1002/cctc.202001294日期:2020.12.4A practical route from oxetane or thietane to γ‐(thio)butyrolactone via solvated‐proton‐assisted cobalt‐catalyzed carbonylative ring expansion under syngas atmosphere has been established. A wide variety of γ‐(thio)butyrolactones can be afforded in good to excellent yields. The versatility of this method has been well demonstrated in the synthesis of intermediates towards the natural product Arctigenin
-
Photocatalytic Synthesis of γ-Lactones from Alkenes: High-Resolution Mass Spectrometry as a Tool To Study Photoredox Reactions作者:Ierasia Triandafillidi、Maroula G. Kokotou、Christoforos G. KokotosDOI:10.1021/acs.orglett.7b03256日期:2018.1.5A mild photocatalytic manifold for the synthesis of γ-lactones has been developed. Utilizing Ru(bpy)3Cl2 as the photocatalyst, a cheap and reproducible synthetic protocol for γ-lactones has been introduced. Mechanistic studies revealed the successful monitoring of photocatalytic reactions and radical intermediates via high-resolution mass spectrometry.已经开发了用于合成γ-内酯的温和光催化歧管。利用Ru(bpy)3 Cl 2作为光催化剂,已经引入了廉价且可重现的γ-内酯合成方案。机理研究表明,通过高分辨率质谱已成功监测了光催化反应和自由基中间体。
表征谱图
-
氢谱1HNMR
-
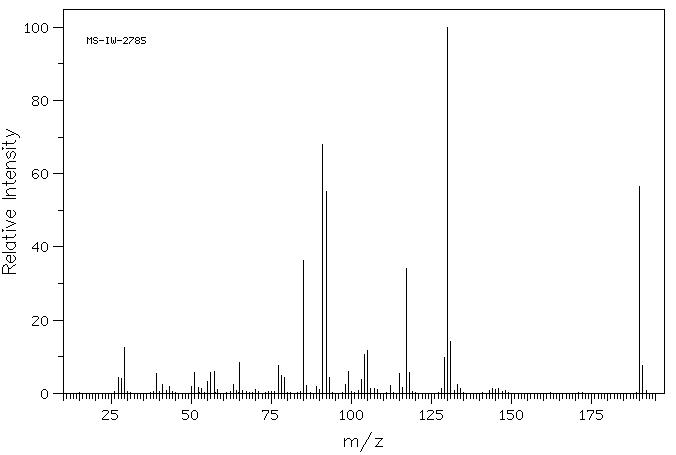
质谱MS
-
碳谱13CNMR
-
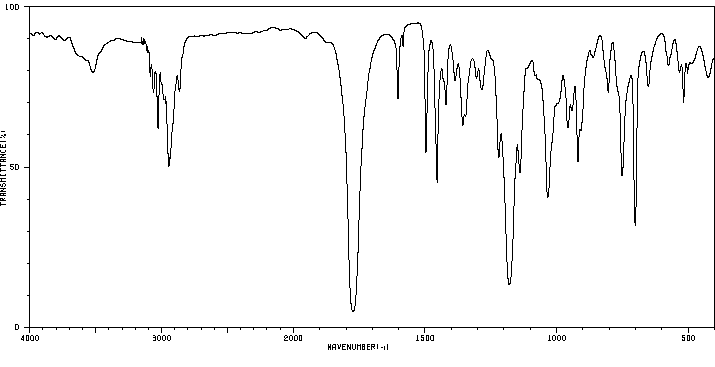
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(+)-(3R)-3-{[叔丁基(二甲基)硅基]氧基}二氢呋喃-2(3H)-酮
龙胆黄碱
龙胆酮胺
高良姜萜内酯
高柠檬酸-gamma-内酯
高普伐他汀内酯二-(叔-丁基二甲基硅烷基)醚
马桑内酯
顺式蒈醛酸内酯
顺式-3,5-二甲基二氢-2H-吡喃-2,6(3H)-二酮
顺式-1,3-环戊烷二甲酸酐
顺式-1,3-环己烷二甲酸酐
阿拉伯酸,2-氨基-2,3,5-三脱氧-3-甲基-,γ-内酯(9CI)
酸,(1S,3R,4R,5R)-3,4-二羟基-7-羰基-6-氧杂二环[3.2.1]辛-1-基2,2,2-三氯乙基酯碳
辛伐他汀4'-甲基醚
辛伐他汀
软木三萜酮3,4-内酯
试剂Menthide
试剂6-Allyl-epsilon-caprolactone
表洛伐他汀
蜂毒
藻酸钠
薇甘菊内酯
葡醛内酯
葡庚糖酸内酯
葡庚糖酸內酯
莫那可林X
莫那可林L
莫那可林J
脱氢抗坏血酸
聚乌拉坦
聚(epsilon-己内酯-delta-戊内酯)
羟基马桑毒内酯
羟基蓍含蓍素
羟基己酸内酯与2,2-二甲基-1,3-丙二醇的聚合物
美伐他汀
绵毛马兜铃内酯
糖质酸-1,4-内酯
穿心莲内酯
科立内脂二醇
硫丹内酯
石蚕苷A
甲酰辛伐他汀
甲瓦龙酸内酯-D4
甲瓦龙酸内酯-D3
甲瓦龙酸内酯-1-13C
甲瓦龙酸内酯-1,2-13C2
甲瓦龙酸内酯
甲基丙烯酸甲瓦龙酸内酯
甲基[(1S,5R,6R)-3-氧代-2-氧杂双环[3.2.1]辛-6-基]乙酸酯
瑞舒伐他汀杂质113