trans-1,4-menthane | 1678-82-6
中文名称
——
中文别名
——
英文名称
trans-1,4-menthane
英文别名
p-menthane;trans-p-menthane
CAS
1678-82-6
化学式
C10H20
mdl
——
分子量
140.269
InChiKey
CFJYNSNXFXLKNS-MGCOHNPYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-86.3°C
-
沸点:170 °C
-
密度:0,79 g/cm3
-
闪点:38°C
-
LogP:5.250 (est)
计算性质
-
辛醇/水分配系数(LogP):3.47
-
重原子数:10.0
-
可旋转键数:1.0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0.0
-
氢给体数:0.0
-
氢受体数:0.0
安全信息
-
安全说明:S16
-
危险类别码:R10
-
海关编码:2902199090
-
危险品运输编号:UN 3295
SDS
上下游信息
反应信息
-
作为反应物:描述:trans-1,4-menthane 在 monooxygenase P450-BM3 (A328F) mutant 、 nicotinamide adenine dinucleotide phosphate 作用下, 反应 3.0h, 生成 氢化松油醇参考文献:名称:P450-catalyzed regio- and stereoselective oxidative hydroxylation of disubstituted cyclohexanes: creation of three centers of chirality in a single CH-activation event摘要:Wild-type P450-BM3 is able to catalyze in a highly regio- and diastereoselective manner the oxidative hydroxylation of non-activated disubstituted cyclohexane derivatives lacking any functional groups, including cis- and trans-1,2-dimethylcyclohexane, cis- and trans-1,4-dimethylcyclohexane, and trans-1,4-methylisopropylcyclohexane. In all cases except chiral trans-1,2-dimethylcyclohexane as substrate, the single hydroxylation event at a methylene group induces desymmetrization with simultaneous creation of three centers of chirality. Certain mutants increase selectivity, setting the stage for future directed evolution work. (C) 2014 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2014.11.067
-
作为产物:描述:参考文献:名称:Optical Activity in Compounds Containing Deuterium. I. 2,3-Dideutero-trans-Menthane摘要:DOI:10.1021/ja01173a073
文献信息
-
A General Copper Catalyst for Photoredox Transformations of Organic Halides作者:Bastien Michelet、Christopher Deldaele、Sofia Kajouj、Cécile Moucheron、Gwilherm EvanoDOI:10.1021/acs.orglett.7b01518日期:2017.7.7irradiation in the presence of catalytic amounts of [(DPEphos)(bcp)Cu]PF6 and an amine, a range of unactivated aryl and alkyl halides were shown to be smoothly activated through a rare Cu(I)/Cu(I)*/Cu(0) catalytic cycle. This complex efficiently catalyzes a series of radical processes, including reductions, cyclizations, and direct arylation of arenes.
-
Reduction of sulfonates and aromatic compounds with the NiCl2·2H2OLi-arene (cat.) combination作者:Gabriel Radivoy、Francisco Alonso、Miguel YusDOI:10.1016/s0040-4020(99)00893-5日期:1999.12A series of alkyl mesylates, dimesylates and alkyl or aryl triflates have been reduced to the corresponding hydrocarbons with nickel(II) chloride dihydrate, an excess of lithium powder and a catalytic amount of DTBB (10 mol%) in THF at room temperature. This methodology applied to enol triflates allowed the preparation of olefins or alkanes depending on the amount of the Ni(II) salt used. The reduction
-
A General Copper-based Photoredox Catalyst for Organic Synthesis: Scope, Application in Natural Product Synthesis and Mechanistic Insights作者:Christopher Deldaele、Bastien Michelet、Hajar Baguia、Sofia Kajouj、Eugenie Romero、Cecile Moucheron、Gwilherm EvanoDOI:10.2533/chimia.2018.621日期:——available catalysts based on inexpensive, environmentally benign base metals are therefore strongly needed. Furthermore, expanding the toolbox of methods based on photoredox catalysis will facilitate the discovery of new light-mediated transformations. This article details the use of a simple copper complex which, upon activation with blue light, can initiate a broad range of radical reactions.有机转化大致可分为四类,包括阳离子反应、阴离子反应、周环反应和自由基反应。尽管几十年来人们都知道最后一类可以提供非常有效的合成途径,但它长期以来一直受到有毒试剂需求的阻碍,这在很大程度上限制了它对化学合成的影响。随着产生自由基物质的新概念的引入,这种情况已经结束,光氧化还原催化——它仅依赖于使用可在可见光照射下激活的催化剂——当然是最有效的一种。最先进的催化剂主要依赖于钌和铱络合物以及有机染料的使用,这仍然在很大程度上限制了它们在化学过程中的广泛应用:因此,迫切需要基于廉价、环境友好的贱金属的替代现成催化剂。此外,扩展基于光氧化还原催化的方法工具箱将有助于发现新的光介导转化。本文详细介绍了简单的铜络合物的使用,该络合物在蓝光激活后可以引发广泛的自由基反应。
-
Heterogeneous Supramolecular Catalysis through Immobilization of Anionic M<sub>4</sub>L<sub>6</sub> Assemblies on Cationic Polymers作者:Hiroyuki Miyamura、Robert G. Bergman、Kenneth N. Raymond、F. Dean TosteDOI:10.1021/jacs.0c09556日期:2020.11.11selectivity and activity through specific host-guest interactions work under homogeneous conditions, enzymes in nature can operate under heterogeneous conditions as membrane-bound enzymes. In order to develop such a heterogeneous system, an immobilized chiral supramolecular cluster Ga416 (2) was introduced into cross-linked polymers with cationic functionalities. These heterogeneous supramolecular catalysts尽管目前开发的大多数超分子催化剂通过特定的主客体相互作用以独特的选择性和活性模拟酶促反应,但在均相条件下工作,自然界中的酶可以在非均相条件下作为膜结合酶运行。为了开发这种异质系统,将固定的手性超分子簇 Ga416 (2) 引入具有阳离子官能团的交联聚合物中。这些非均相超分子催化剂用于 aza-Prins 和 aza-Cope 反应,并成功应用于连续流动反应。他们表现出很高的耐用性,并在很长一段时间内保持高营业额。与大多数多相均相催化剂的例子形成鲜明对比,与相应的可溶性簇催化剂相比,新开发的催化剂表现出更高的活性和稳定性。还固定了对映体富集的簇以实现不对称催化,并且在回收和再利用实验期间以及在连续流动过程中保持负载型手性催化剂的活性和对映体选择性。值得注意的是,聚合物中铵阳离子的结构影响稳定性、反应性和对映选择性,这与聚合物载体中的阳离子部分与作为外面体保护壳的簇相互作用从而影响其催化性能的
-
Cob(I)alamin als Katalysator. 6. Mitteilung [1]. Bildung und Fragmentierung von Alkylcobalaminen, ein Gleichgewichtsprozess zwischen nukleophiler Addition und reduktiver Fragmentierung
表征谱图
-
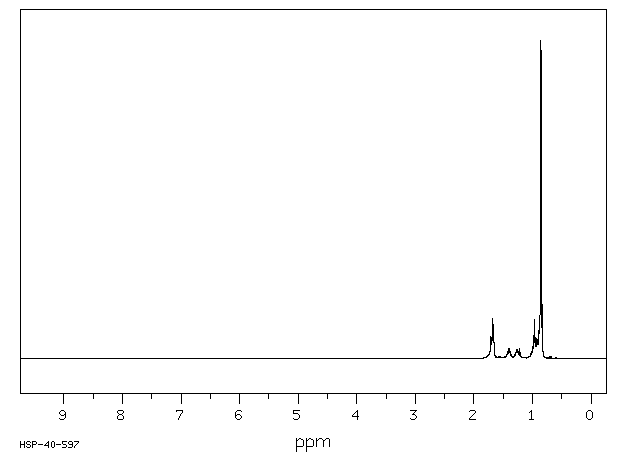
氢谱1HNMR
-
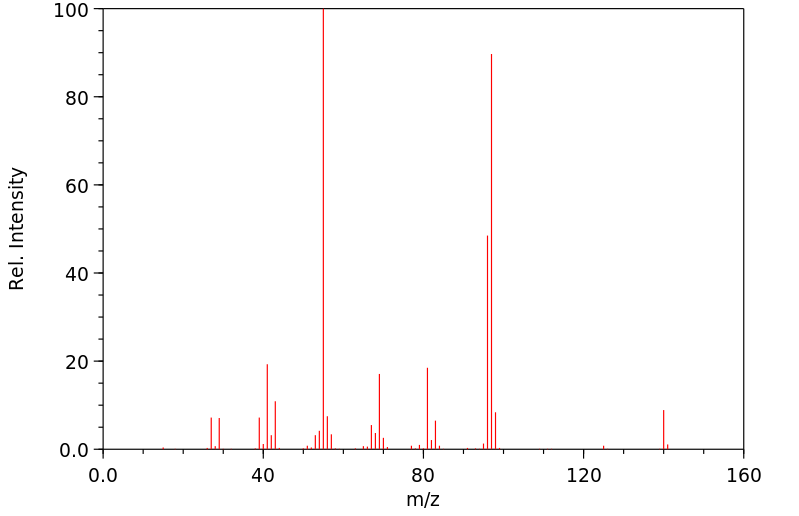
质谱MS
-
碳谱13CNMR
-
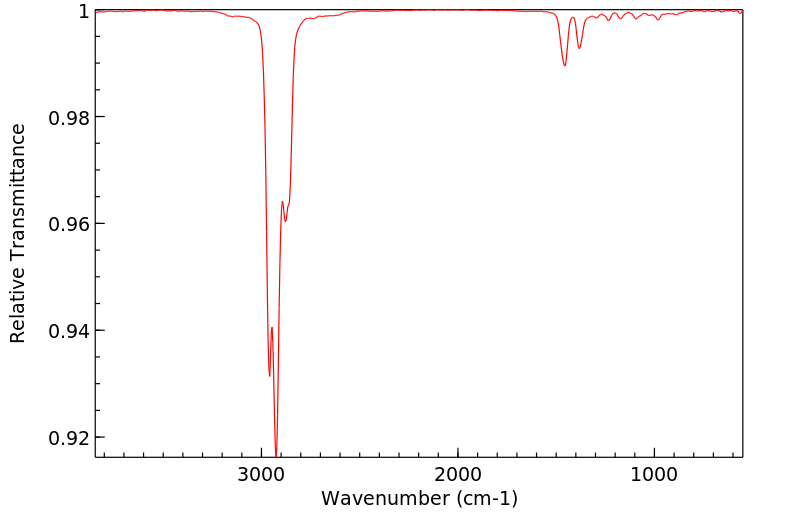
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸