乙酰戊二酸二乙酯 | 1501-06-0
中文名称
乙酰戊二酸二乙酯
中文别名
——
英文名称
diethyl 2-acetylglutarate
英文别名
diethyl 2-acetylpentanedioate
CAS
1501-06-0
化学式
C11H18O5
mdl
MFCD00009158
分子量
230.261
InChiKey
NNOGMCQLKMLNPL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:154 °C/11 mmHg (lit.)
-
密度:1.071 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
稳定性/保质期:
如果按照规格使用和储存,是不会分解的,也没有已知危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:16
-
可旋转键数:9
-
环数:0.0
-
sp3杂化的碳原子比例:0.727
-
拓扑面积:69.7
-
氢给体数:0
-
氢受体数:5
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2918300090
-
储存条件:保持贮藏器密封,并将其存放在阴凉、干燥的地方。确保工作间具备良好的通风或排气装置。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Diethyl 2-acetylglutarate
CAS-No. : 1501-06-0
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C11H18O5
Molecular Weight : 230,26 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Normal measures for preventive fire protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection not required. For nuisance exposures use type OV/AG (US) or type ABEK
(EU EN 14387) respirator cartridges. Use respirators and components tested and approved under
appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 154 °C at 15 hPa - lit.
boiling range
g) Flash point > 113,00 °C - closed cup
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,071 g/mL at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
acids, Bases, Oxidizing agents, Reducing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (+/-)-4-ethoxycarbonyl-5-oxohexanoic acid 42161-08-0 C9H14O5 202.207 —— 2-acetyl-2-methyl-glutaric acid diethyl ester 58997-95-8 C12H20O5 244.288 —— diethyl 2-acetyl-2-(2-formylethyl)glutarate —— C14H22O6 286.325 3-乙酰基戊烷-1,3,5-三羧酸三乙酯 tris-ethyl 3-acetylpentane-1,3,5-tricarboxylate 72948-75-5 C16H26O7 330.378 —— diethyl 2-(bromoacetyl)glutarate 41845-25-4 C11H17BrO5 309.157 —— 2-acetylglutaric acid —— C7H10O5 174.153
反应信息
-
作为反应物:参考文献:名称:Hamlin; Hartung, Journal of Biological Chemistry, 1942, vol. 145, p. 352摘要:DOI:
-
作为产物:描述:参考文献:名称:通过转氨酶催化的动态动力学拆分化学酶立体选择合成具有两个手性中心的γ-或δ-内酰胺摘要:通过动态动力学拆分策略证明了具有两个手性中心的内酰胺的生物催化立体选择性合成。检查了五种转氨酶,以化学合成取代的γ-或δ-酮酸酯进行氨基转移。(R)-和(S)-选择酶的应用导致相应的手性胺具有中等至高的非对映异构体比率和出色的对映异构体过量,随后在某些情况下形成了γ-或δ-内酰胺。优化了反应条件,包括pH,助溶剂和胺供体与受体的比例。立体选择性生物转氨反应在半制备规模下进行,成功地以46–70%的分离产率成功生成了相应的内酰胺或胺,非对映异构体比例高达99:1,> 99%ee值。这项研究代表了一种重要的具有两个手性中心的重要γ-和δ-内酰胺生物催化合成方法。DOI:10.1002/cctc.202001142
文献信息
-
A focused structure–activity relationship study of psoralen-based immunoproteasome inhibitors作者:Eva Shannon Schiffrer、Izidor Sosič、Andrej Šterman、Janez Mravljak、Irena Mlinarič Raščan、Stanislav Gobec、Martina GobecDOI:10.1039/c9md00365g日期:——
SAR exploration at a single position of the psoralen ring led to improved selectivity to the chymotrypsin-like (β5i) subunit of the immunoproteasome.
-
Five Roads That Converge at the Cyclic Peroxy-Criegee Intermediates: BF<sub>3</sub>-Catalyzed Synthesis of β-Hydroperoxy-β-peroxylactones作者:Vera A. Vil’、Gabriel dos Passos Gomes、Maria V. Ekimova、Konstantin A. Lyssenko、Mikhail A. Syroeshkin、Gennady I. Nikishin、Igor V. Alabugin、Alexander O. Terent’evDOI:10.1021/acs.joc.8b02218日期:2018.11.2actones from silyl enol ethers, enol acetates, and cyclic acetals confirm that the β-peroxylactones indeed correspond to a deep energy minimum that connects a variety of the interconverting oxygen-rich species at this combined potential energy surface. The target β-hydroperoxy-β-peroxylactones were synthesized from β-ketoesters, and their silyl enol ethers, alkyl enol ethers, enol acetates, and cyclic我们发现通过BF 3催化的各种无环前体,β-酮酸酯及其甲硅烷基烯醇醚,烷基烯醇醚,烯醇乙酸酯和环状缩醛与H 2 O的BF 3催化环化反应,可合成获得β-氢过氧-β-过氧内酯。2个。引人注目的是,与起始原料的选择无关,这些反应收敛于相同的β-氢过氧-β-过氧内酯产物,即先前难以捉摸的Baeyer-Villiger反应的环状Criegee中间体的过氧类似物。由甲硅烷基烯醇醚,烯醇乙酸酯和环状缩醛形成β-氢过氧-β-过氧内酯的计算热力学参数证实,β-过氧内酯确实对应于连接各种相互转化的富氧物种的最低能量最小值。在这个组合的势能面。由β-酮酸酯合成了目标β-氢过氧-β-过氧内酯,并以30-96%的收率获得了它们的甲硅烷基烯醇醚,烷基烯醇醚,烯醇乙酸酯和环状缩醛。这些反应在温和的条件下进行,即使在可以预期替代氧化途径的情况下,也可以选择性地形成多种选择性选择性形成的β-氢过氧-β-过氧内酯。这些β-
-
[EN] BENZOPYRANE AND IMIDAZOLE DERIVATIVES USEFUL FOR THE STABILIZATION OF AMYLOIDOGENIC IMMUNOGLOBULIN LIGHT CHAINS<br/>[FR] DÉRIVÉS DE STABILISATION DE BENZOPYRANE ET D'IMIDAZOLE UTILISÉS POUR LA STABILISATION DE CHAÎNES LÉGÈRES D'IMMUNOGLOBULINES AMYLOÏDOGÉNIQUES申请人:SCRIPPS RESEARCH INST公开号:WO2020205683A1公开(公告)日:2020-10-08In immunoglobulin light chain amyloidosis (AL), the unique antibody light chain (LC) protein that is secreted by monoclonal plasma cells in each patient misfolds and/or aggregates, a process leading to organ degeneration. For treating AL patients, such as those with substantial cardiac involvement who have difficulty tolerating existing chemotherapy regimens, provided herein are small molecule compounds of Formula Ia, Formula Ib, and Formula II that are kinetic stabilizers of the native dimeric structure of full-length LCs, which compounds can slow or stop the amyloidogenicity cascade at its origin.
-
Biosynthesis of the antitumor antibiotic sparsomycin作者:Ronald J. Parry、Yan Li、Elizabeth Eudy GomezDOI:10.1021/ja00041a007日期:1992.7The biosynthesis of the antitumor antibiotic sparsomycin (1) has been investigated by administration of isotopically labeled precursors to Streptomyces sparsogenes var. sparsogenes. These studies indicated that the dithioacetal moiety (2) of sparsomycin is derived from L-cysteine via the intermediacy of 5-methylcysteine and S-(methylthiomethyl)cysteine, with reduction of the carboxyl group of 5-(m
-
Assembly of Four Diverse Heterocyclic Libraries Enabled by Prins Cyclization, Au-Catalyzed Enyne Cycloisomerization, and Automated Amide Synthesis作者:Jiayue Cui、David I. Chai、Christopher Miller、Jason Hao、Christopher Thomas、JingQi Wang、Karl A. Scheidt、Sergey A. KozminDOI:10.1021/jo301061r日期:2012.9.7simple one-flask operation starting with readily available amines, ketoesters, and unsaturated anhydrides. The use of tetrahydropyran-containing ketoesters, which were rapidly assembled by our Prins cyclization protocol, enabled efficient fusion of pyran and piperidinone cores. A newly developed Au(I)-catalyzed cycloisomerization of alkyne-containing enamides further expanded heterocyclic diversity by
表征谱图
-
氢谱1HNMR
-
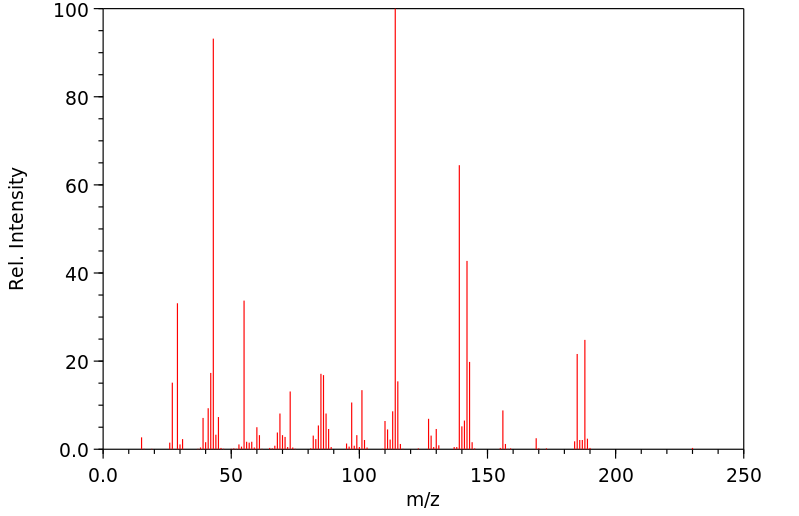
质谱MS
-
碳谱13CNMR
-
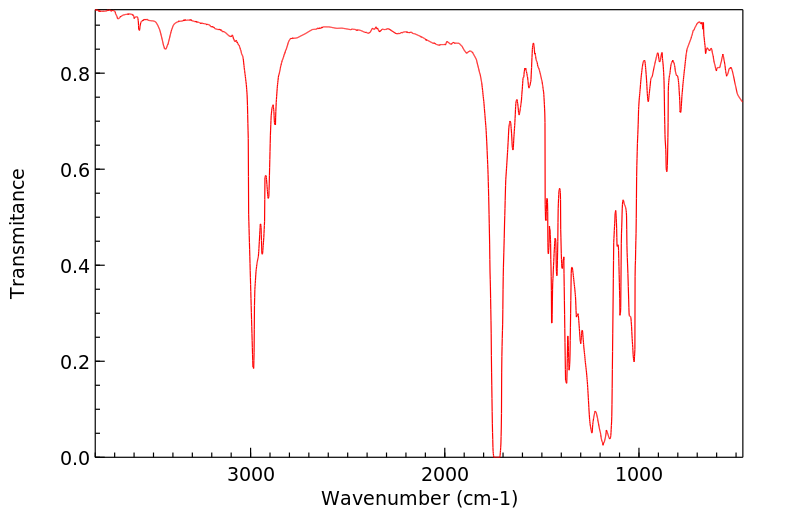
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰基乙酸
顺-3-己烯-1-丙酮酸
青霉酸
钠氟草酰乙酸二乙酯
醚化物
酮霉素
辛酸,2,4-二羰基-,乙基酯
草酸乙酯钠盐
草酰乙酸二乙酯钠盐
草酰乙酸二乙酯
草酰乙酸
草酰丙酸二乙酯
苯乙酰丙二酸二乙酯
苯丁酸,b-羰基-,2-丙烯基酯
聚氧化乙烯
羟基-(3-羟基-2,3-二氧代丙基)-氧代鏻
磷酸二氢2-{(E)-2-[4-(二乙胺基)-2-甲基苯基]乙烯基}-1,3,3-三甲基-3H-吲哚正离子
碘化镝
硬脂酰乙酸乙酯
甲氧基乙酸乙酯
甲氧基乙酰乙酸酯
甲基氧代琥珀酸二甲盐
甲基4-环己基-3-氧代丁酸酯
甲基4-氯-3-氧代戊酸酯
甲基4-氧代癸酸酯
甲基4-氧代月桂酸酯
甲基4-(甲氧基-甲基磷酰)-2,2,4-三甲基-3-氧代戊酸酯
甲基3-羰基-2-丙酰戊酸酯
甲基3-氧代十五烷酸酯
甲基2-氟-3-氧戊酯
甲基2-氟-3-氧代己酸酯
甲基2-氟-3-氧代丁酸酯
甲基2-乙酰基环丙烷羧酸酯
甲基2-乙酰基-4-甲基-4-戊烯酸酯
甲基2-乙酰基-2-丙-2-烯基戊-4-烯酸酯
甲基2,5-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代丁酸酯
甲基1-异丁酰基环戊烷羧酸酯
甲基1-乙酰基环戊烷羧酸酯
甲基1-乙酰基环丙烷羧酸酯
甲基1-乙酰基-2-乙基环丙烷羧酸酯
甲基(2Z,4E,6E)-2-乙酰基-7-(二甲基氨基)-2,4,6-庚三烯酸酯
甲基(2S)-2-甲基-4-氧代戊酸酯
甲基(1S,2R)-2-乙酰基环丙烷羧酸酯
甲基(1R,2R)-2-乙酰基环丙烷羧酸酯
瑞舒伐他汀杂质
瑞舒伐他汀杂质
环氧乙烷基甲基乙酰乙酸酯
环戊戊烯酸,Β-氧代,乙酯