2,4-二甲氧基苄醇 | 7314-44-5
中文名称
2,4-二甲氧基苄醇
中文别名
2,4-二甲氧基苯甲醇
英文名称
2,4-dimethoxylbenzyl alcohol
英文别名
2,4-dimethoxybenzyl alcohol;(2,4-dimethoxyphenyl)methanol;Dmob-alcohol;1-hydroxymethyl-2,4-dimethoxybenzene
CAS
7314-44-5
化学式
C9H12O3
mdl
——
分子量
168.192
InChiKey
RNKOUSCCPHSCFE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:38-40 °C(lit.)
-
沸点:177-179 °C10 mm Hg(lit.)
-
密度:1.1322 (rough estimate)
-
闪点:>230 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:1.109 (est)
-
稳定性/保质期:
如果按照规范使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:38.7
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2909499000
-
危险品运输编号:NONH for all modes of transport
-
储存条件:密封保存在阴凉干燥的环境中。
SDS
1.1 产品标识符
: 2,4-Dimethoxybenzyl alcohol
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H12O3
分子式
: 168.19 g/mol
分子量
成分 浓度
2,4-Dimethoxybenzyl alcohol
-
化学文摘编号(CAS No.) 7314-44-5
EC-编号 230-775-2
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 38 - 40 °C - lit.
f) 起始沸点和沸程
177 - 179 °C 在 13 hPa - lit.
g) 闪点
> 113.00 °C - 闭杯
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
酸, 酰氯, 酸酐, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 2,4-Dimethoxybenzyl alcohol
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H12O3
分子式
: 168.19 g/mol
分子量
成分 浓度
2,4-Dimethoxybenzyl alcohol
-
化学文摘编号(CAS No.) 7314-44-5
EC-编号 230-775-2
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 38 - 40 °C - lit.
f) 起始沸点和沸程
177 - 179 °C 在 13 hPa - lit.
g) 闪点
> 113.00 °C - 闭杯
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
酸, 酰氯, 酸酐, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二甲氧基苯甲酸 2,4 dimethoxybenzoic acid 91-52-1 C9H10O4 182.176 2,4-二甲氧基苯甲酸甲酯 methyl 2, 4-dimethoxybenzoate 2150-41-6 C10H12O4 196.203 2,4-二甲氧基苯甲醛 2,4-Dimethoxybenzaldehyde 613-45-6 C9H10O3 166.177 2,4-二羟基苯甲酸 4-hydroxysalicylic acid 89-86-1 C7H6O4 154.122 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 O-(2,4-二甲氧基苄基)羟胺 O-(2,4-dimethoxybenzyl)hydroxylamine 216067-66-2 C9H13NO3 183.207 —— 2,4-dimethoxybenzyl ether —— C18H22O5 318.37 2,4-二甲氧基苯甲酸 2,4 dimethoxybenzoic acid 91-52-1 C9H10O4 182.176 —— 2,4-dimethoxybenzyl acetate 880879-37-8 C11H14O4 210.23 —— 2,4-dimethoxybenzyl mesylate 187979-64-2 C10H14O5S 246.284 2,4-二甲氧基苯甲酸甲酯 methyl 2, 4-dimethoxybenzoate 2150-41-6 C10H12O4 196.203 2,4-二甲氧基苯甲醛 2,4-Dimethoxybenzaldehyde 613-45-6 C9H10O3 166.177 —— (2,4-dimethoxyphenyl)methyl 2,2,2-trichloroacetimidate 1207538-85-9 C11H12Cl3NO3 312.58 2,4-二甲氧基苄基溴 2,4-dimethoxybenzyl bromide 161919-74-0 C9H11BrO2 231.089 2,4-二甲氧基苯甲腈 2,4-dimethoxybenzonitrile 4107-65-7 C9H9NO2 163.176 1,3-二甲氧基-4-乙炔基苯 1,3-dimethoxy-4-ethynylbenzene 77336-36-8 C10H10O2 162.188 (2,4-二甲氧基苯基)甲硫醇 2,4-dimethoxybenzyl thiol 114719-65-2 C9H12O2S 184.259 —— 4-[(2,4-dimethoxyphenyl)methoxy]-4-oxobutanoic acid 1282955-16-1 C13H16O6 268.266 2,4-二甲氧基苄基氯 1-(chloromethyl)-2,4-dimethoxybenzene 55791-52-1 C9H11ClO2 186.638 —— O-(2,4-dimethoxybenzyl)-N-(2,4,6-trimethoxybenzyl)hydroxylamine 215607-30-0 C19H25NO6 363.411 —— 2,4-dimethoxybenzyl 4-methoxyphenylacetate 258875-95-5 C18H20O5 316.354 —— 1-(azidomethyl)-2,4-dimethoxybenzene —— C9H11N3O2 193.205 —— di-(o,p-dimethoxybenzyl)-N,N-diisopropylphosphoramidite 1069059-92-2 C24H36NO6P 465.527 —— bis(2,4-dimethoxyphenyl)methane 72046-68-5 C17H20O4 288.343 - 1
- 2
反应信息
-
作为反应物:描述:2,4-二甲氧基苄醇 在 三溴化磷 、 sodium hydroxide 作用下, 以 乙醇 、 二氯甲烷 、 乙腈 为溶剂, 反应 4.08h, 生成 7-[3-(benzylideneamino)methyl-4-(2',4'-dimethxoybenzyl-oxyimino)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid参考文献:名称:Synthesis and in vitro antibacterial activity of gemifloxacin derivatives containing a substituted benzyloxime moiety摘要:A series of novel gemifloxacin (GMFX) derivatives containing a substituted benzyloxime moiety with remarkable improvement in lipophilicity were synthesized. The target compounds evaluated for their in vitro antibacterial activity against representative strains. Our results reveal that most of the target compounds have considerable potency against all of the tested Gram-positive strains including MRSA and MRSE (MIC: <0.008-8 mu g/mL), although they are generally less active than the references against the Gram-negative strains. In particular, compound 111 (MIC: <0.008-4 mu g/mL) was found to be 8-2048 and 2-128 times more potent than levofloxacin (LVFX) and GMFX against the Gram-positive strains, respectively. Moreover, against MRSA clinical isolates, 111 (MIC90: 1 mu g/mL) is 8-fold more active than GMFX, and 2-fold more active than GMFX and moxifloxacin against MRSE clinical isolates (MIC90: 4 mu g/mL). Crown Copyright (C) 2012 Published by Elsevier Masson SAS. All rights reserved.DOI:10.1016/j.ejmech.2012.07.010
-
作为产物:描述:2,4-二甲氧基苯甲酸 在 sodium tetrahydroborate 、 sodium sulfate 、 3,4,5-三氟苯硼酸 作用下, 以 四氢呋喃 为溶剂, 反应 10.0h, 以99%的产率得到2,4-二甲氧基苄醇参考文献:名称:使用3,4,5-三氟苯基硼酸和硼氢化钠将一氧化碳非常简单,方便,温和地一锅还原为醇摘要:由羧酸和3,4,5-三氟苯基硼酸原位形成的酰氧基硼中间体在THF中与硼氢化钠反应生成醇,收率高至高。DOI:10.1016/s0040-4039(03)00035-2
-
作为试剂:描述:4-(6-氯-4-碘吡啶-2-基)吗啉 在 四(三苯基膦)钯 、 2,4-二甲氧基苄醇 、 potassium tert-butylate 、 potassium carbonate 作用下, 以 1,4-二氧六环 、 乙醇 、 水 、 甲苯 为溶剂, 反应 5.0h, 生成 4-[6-chloro-4-(2-methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)-2-pyridyl]morpholine参考文献:名称:WO2019038384A5摘要:公开号:WO2019038384A5
文献信息
-
SULFOXIMINE SUBSTITUTED QUINAZOLINES FOR PHARMACEUTICAL COMPOSITIONS申请人:BLUM Andreas公开号:US20140135309A1公开(公告)日:2014-05-15This invention relates to novel sulfoximine substituted quinazoline derivatives of formula I wherein Ar, R 1 and R 2 are as defined herein, and their use as MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) kinase inhibitors, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment or amelioration of MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) mediated disorders.
-
[EN] SULFOXIMINE SUBSTITUTED QUINAZOLINES FOR PHARMACEUTICAL COMPOSITIONS<br/>[FR] QUINAZOLINES SUBSTITUÉES PAR SULFOXIMINE POUR COMPOSITIONS PHARMACEUTIQUES申请人:BOEHRINGER INGELHEIM INT公开号:WO2014072244A1公开(公告)日:2014-05-15This invention relates to novel sulfoximine substituted quinazoline derivatives of formula (I), wherein Ar, R1 and R2 are as defined in the description and claims, and their use as MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) kinase inhibitors, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment or amelioration of MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) mediated disorders.
-
[EN] IMIDAZOTHIADIAZOLE AND IMIDAZOPYRAZINE DERIVATIVES AS PROTEASE ACTIVATED RECEPTOR 4 (PAR4) INHIBITORS FOR TREATING PLATELET AGGREGATION<br/>[FR] DÉRIVÉS D'IMIDAZOTHIADIAZOLE ET D'IMIDAZOPYRAZINE UTILISÉS COMME INHIBITEURS DU RÉCEPTEUR 4 ACTIVÉ PAR UNE PROTÉASE (PAR4) POUR LE TRAITEMENT DE L'AGRÉGATION PLAQUETTAIRE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2013163279A1公开(公告)日:2013-10-31The present invention provides thiazole compounds of Formula I wherein W, Y, R0, R2, R4, R5, R6, R7, X1, X2, X3 and X4 are as defined herein, or a stereoisomer, tautomer, pharmaceutically acceptable salt, prodrug ester or solvate form thereof, wherein all of the variables are as defined herein. These compounds are inhibitors of platelet aggregation and thus can be used as medicaments for treating or preventing thromboembolic disorders.
-
Efficient and Convenient Procedure for Protection of Hydroxyl Groups to the THP, THF and TMS Ethers and Oxidation of these Ethers to their Aldehydes or Ketones in [BPy]FeCl<sub>4</sub> as a Low Cost Room Temperature Ionic Liquid作者:Ahmad R. Khosropour、Mohammad M. Khodaei、Sattar GhaderiDOI:10.1515/znb-2006-0313日期:2006.3.1
Abstract Alcohols were converted to the corresponding THP, THF or TMS ethers in high to excellent yields in 1-n-butylpyridinium chloroferrate media as a stable and low cost room temperature ionic liquid. In addition, oxidation of these ethers to their aldehydes or ketones without any overoxidation reactions in this ionic liquid was also performed.
-
Product Studies and Laser Flash Photolysis on Alkyl Radicals Containing Two Different β-Leaving Groups Are Consonant with the Formation of an Olefin Cation Radical作者:Brian C. Bales、John H. Horner、Xianhai Huang、Martin Newcomb、David Crich、Marc M. GreenbergDOI:10.1021/ja0042938日期:2001.4.1hydrogen atom transfer to the oxygen of the olefin cation radical, followed by deprotonation. Laser flash photolysis experiments indicate that reaction between 5 and 1,4-cyclohexadiene occurs with a rate constant of approximately 6 x 10(5) M(-1) s(-1). 2,2-Dimethoxy-1-phenylpropane (18) is observed as a minor product. Laser flash photolysis experiments place an upper limit on methanol trapping of 51-溴-2-甲氧基-1-苯基丙-2-基 (3) 和 2-甲氧基-1-苯基-1-二苯基磷酸丙-2-基 (4) 是在混合物中各自 PTOC 前体的连续光解下生成的乙腈和甲醇。自由基在β-位进行取代基的杂裂断裂以产生烯烃阳离子自由基(5)。Z-2-甲氧基-1-苯基丙烯 (15) 是在 1,4- 环己二烯存在下形成的主要产物,据信是由氢原子转移到烯烃阳离子自由基的氧上,然后去质子化的结果。激光闪光光解实验表明 5 和 1,4-环己二烯之间的反应以大约 6 x 10(5) M(-1) s(-1) 的速率常数发生。观察到 2,2-二甲氧基-1-苯基丙烷 (18) 作为次要产物。激光闪光光解实验在 k <1 x 10(3) M(-1) s(-1) 处将甲醇捕获的上限设置为 5,并且不提供除 5 以外的反应性中间体的形成的任何证据。使用两种含有不同离去基团的 PTOC 前体生成一个共同的烯烃阳离子自由基使
表征谱图
-
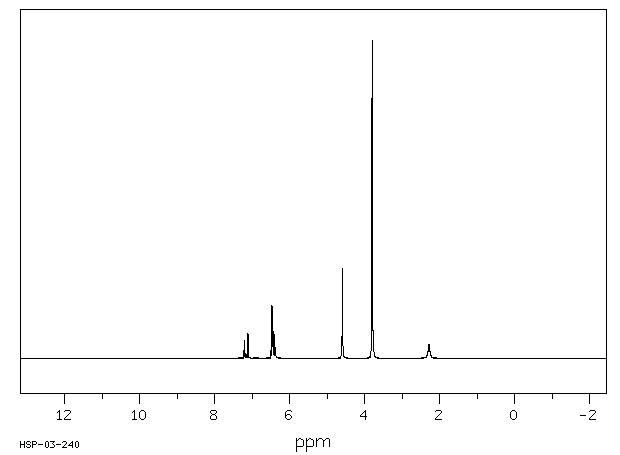
氢谱1HNMR
-
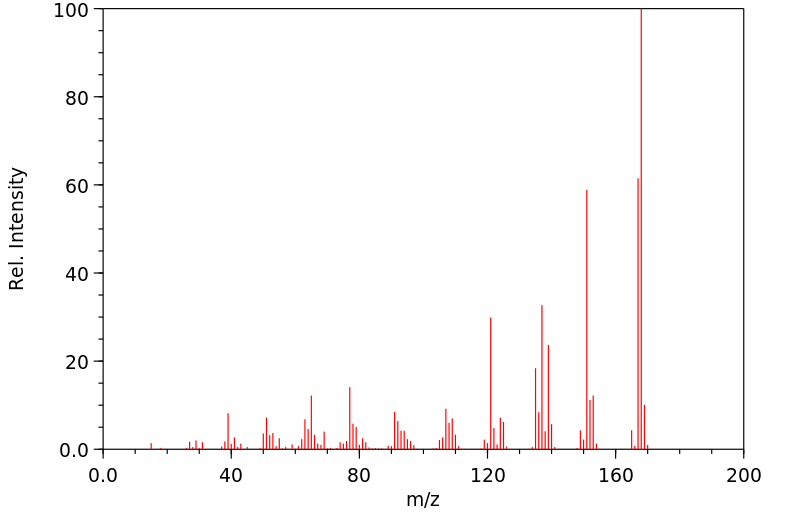
质谱MS
-
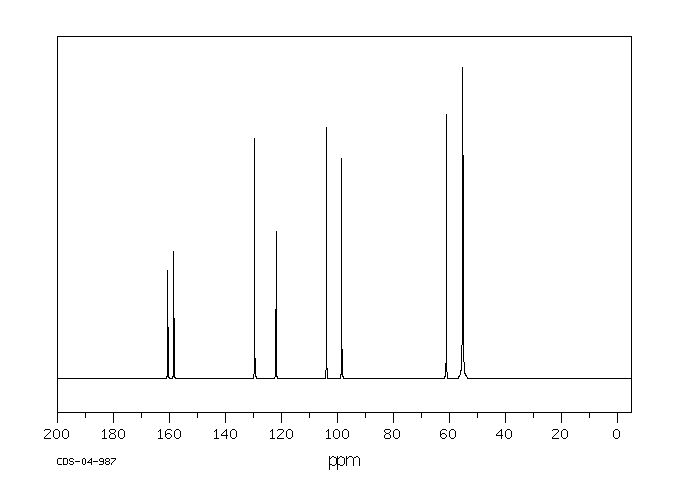
碳谱13CNMR
-
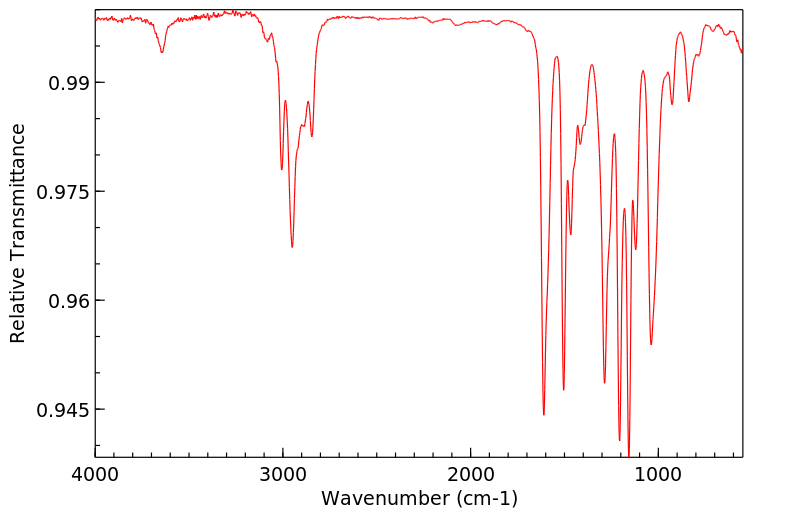
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫