氢醌-О,О'-二乙酸 | 2245-53-6
中文名称
氢醌-О,О'-二乙酸
中文别名
氢醌-О,О′-二乙酸;氢醌-O,O'-二乙酸;对苯二酚-O,O'-二乙酸;氢醌-O,O"-二乙酸
英文名称
2,2'-[1,4-phenylenebis(oxy)]bisacetic acid
英文别名
hydroquinone-O,O'-diacetic acid;hydroquinone-o,o’-diacetic acid;Acetic acid, 2,2'-[1,4-phenylenebis(oxy)]bis-;2-[4-(carboxymethoxy)phenoxy]acetic acid
CAS
2245-53-6
化学式
C10H10O6
mdl
MFCD00016816
分子量
226.186
InChiKey
DNXOCFKTVLHUMU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:248-251 °C
-
沸点:287.79°C (rough estimate)
-
密度:1.2437 (rough estimate)
-
稳定性/保质期:
避免让氧化物直接接触。
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:16
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:93.1
-
氢给体数:2
-
氢受体数:6
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2918990090
-
储存条件:请将容器密封,并将其存放在紧密封装的容器中,保管在阴凉、干燥的地方。
SDS
| Name: | Hydroquinone-O O -diacetic acid Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2245-53-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2245-53-6 | Hydroquinone-O,O'-diacetic acid | 218-834-0 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2245-53-6: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 248 - 251 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H10O6
Molecular Weight: 226.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2245-53-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Hydroquinone-O,O'-diacetic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 2245-53-6: No information available.
Canada
CAS# 2245-53-6 is listed on Canada's NDSL List.
CAS# 2245-53-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2245-53-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-羟基苯氧乙酸 (4-hydroxyphenoxy)acetic acid 1878-84-8 C8H8O4 168.149 —— 1,4-bis(methyloxycarbonylmethylenoxy)benzene 80791-19-1 C12H14O6 254.24 —— diethyl 2,2'-(phen-1,4-ylenedioxy)bis(acetate) 5897-78-9 C14H18O6 282.293 —— (4-methoxycarbonylmethoxycarbonylmethoxy-phenoxy)acetic acid methoxycarbonylmethyl ester 936257-19-1 C16H18O10 370.313 —— 2,2'-(biphenyl-4,4'-diylbis(oxy))diacetic acid 30596-04-4 C16H14O6 302.284 —— 2-{2-[4-(1-Methoxycarbonyl-ethoxycarbonylmethoxy)-phenoxy]-acetoxy}-propionic acid methyl ester 936257-20-4 C18H22O10 398.367 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,4-bis(methyloxycarbonylmethylenoxy)benzene 80791-19-1 C12H14O6 254.24 —— diethyl 2,2'-(phen-1,4-ylenedioxy)bis(acetate) 5897-78-9 C14H18O6 282.293 —— (1,4-phenylenedioxy)di(acetyl chloride) 1889-01-6 C10H8Cl2O4 263.077 —— (4-methoxycarbonylmethoxycarbonylmethoxy-phenoxy)acetic acid methoxycarbonylmethyl ester 936257-19-1 C16H18O10 370.313 —— 2-{2-[4-(1-Methoxycarbonyl-ethoxycarbonylmethoxy)-phenoxy]-acetoxy}-propionic acid methyl ester 936257-20-4 C18H22O10 398.367
反应信息
-
作为反应物:描述:参考文献:名称:Ettel et al., Collection of Czechoslovak Chemical Communications, 1950, vol. 15, p. 1050,1064摘要:DOI:
-
作为产物:描述:diethyl 2,2'-(phen-1,4-ylenedioxy)bis(acetate) 在 sodium hydroxide 作用下, 以 四氢呋喃 、 水 为溶剂, 以95.4%的产率得到氢醌-О,О'-二乙酸参考文献:名称:A color-tunable fluorescent pillararene coordination polymer for efficient pollutant detection摘要:一种具有可调颜色的柱芳烃配位聚合物(DCP5-Eu1Tb3),具有引人注目的白光发射特性,并能够在生物平台中检测硝基芳香污染物,已被构建。DOI:10.1039/c9ta13776a
-
作为试剂:描述:2-cyanoethoxy-N,N'-diisopropylamino-(6-N-benzoyl-2',3'-di-O-isobutyryl-β-D-adenyl-5'-yl)phosphine 、 在 吡啶 、 乙酸酐 、 4-二甲氨基吡啶 、 氢醌-О,О'-二乙酸 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 三乙胺 作用下, 以 吡啶 为溶剂, 反应 32.0h, 以34%的产率得到参考文献:名称:定义明确的二磷酸腺苷核糖低聚物的合成摘要:翻译后的蛋白质修饰称为二磷酸腺苷核糖基化(ADPr),可调节多种重要的生物学过程,例如DNA损伤修复和细胞代谢。该修饰也参与致癌作用和衰老过程。因此,更好地了解ADP核糖基化的功能对于开发新型疗法至关重要。为了便于阐明ADPr的生物学特性,必须使用定义明确的聚(ADP-核糖)片段。本文中,我们报告了一种固相合成方法,用于制备长度精确确定的ADP-核糖低聚物。该方法以首次报道的ADP核糖二聚体和三聚体的合成为例。DOI:10.1002/anie.201412283
文献信息
-
Universal building blocks and support media for synthesis of oligonucleotides and their analogs申请人:——公开号:US20040152905A1公开(公告)日:2004-08-05Compounds for the synthesis of oligomeric compounds, particularly oligonucleotides and chemically modified oligonucleotide analogs, are provided. In addition, methods for functionalization of a support medium with a first monomeric subunit and methods for the synthesis of oligomeric compounds utilizing the novel compounds bound to support media are provided.提供用于合成寡聚化合物,特别是寡核苷酸和化学修饰的寡核苷酸类似物的化合物。此外,还提供了将支持介质功能化为第一单体亚基的方法,以及利用与支持介质结合的新化合物合成寡聚化合物的方法。
-
Catalyst-Free Photoredox Addition-Cyclisations: Exploitation of Natural Synergy between Aryl Acetic Acids and Maleimide作者:David W. Manley、Andrew Mills、Christopher O'Rourke、Alexandra M. Z. Slawin、John C. WaltonDOI:10.1002/chem.201304929日期:2014.4.25Suitably functionalised carboxylic acids undergo a previously unknown photoredox reaction when irradiated with UVA in the presence of maleimide. Maleimide was found to synergistically act as a radical generating photoxidant and as a radical acceptor, negating the need for an extrinsic photoredox catalyst. Modest to excellent yields of the product chromenopyrroledione, thiochromenopyrroledione and
-
[EN] POLYNUCLEOTIDE CONSTRUCTS HAVING DISULFIDE GROUPS<br/>[FR] CONSTRUCTIONS POLYNUCLÉOTIDIQUES CONTENANT DES GROUPES DISULFURE申请人:SOLSTICE BIOLOG LTD公开号:WO2015069932A1公开(公告)日:2015-05-14The invention features polynucleotide constructs containing one or more components (i) containing a disulfide linkage, where each of the one or more components is attached to an internudeotide bridging group or a terminal group of the polynucleotide construct, and each of the one or more components (i) contains one or more bulky groups proximal to the disulfide group. The invention also features polynucleotide constructs containing one or more components (i) containing a disulfide linkage, where each of the one or more components (i) is attached to an internudeotide bridging group or a terminal group of the polynucleotide construct, and each of the one or more components (i) contains at least 4 atoms in a chain between the disulfide linkage and the phosphorus atom of the internudeotide bridging group or the terminal group; and where the chain does not contain a phosphate, an amide, an ester, or an alkenylene. The invention also features methods of delivering a polynucleotide to a cell using the polynucleotide constructs of the invention.
-
Novel Fluoride-Labile Nucleobase-Protecting Groups for the Synthesis of 3′(2′)-O-Aminoacylated RNA Sequences作者:Alfred Stutz、Claudia Höbartner、Stefan PitschDOI:10.1002/1522-2675(20000906)83:9<2477::aid-hlca2477>3.0.co;2-9日期:2000.9.6a general approach to a total synthesis of amino-acylated t-RNAs and analogs, we describe the synthesis of stabilized, amino-acylated RNA fragments, which, upon ligation, could lead to amino-acylated t-RNA structures. Novel RNA phosphoramidites with fluoride-labile 2'-O-[(triisopropylsilyl)-oxy]methyl (=tom) sugar-protecting and N-2-[(triisopropylsilyl)oxy]benzyl}oxy}carbonyl (=tboc) base-protecting为了开发一种全合成氨酰化 t-RNA 和类似物的通用方法,我们描述了稳定的氨酰化 RNA 片段的合成,这些片段在连接后可能导致氨酰化 t-RNA 结构. 具有氟化物不稳定的 2'-O-[(三异丙基甲硅烷基)-氧基]甲基 (=tom) 糖保护和 N-2-[(三异丙基甲硅烷基)氧基]苄基}氧基}羰基 (=tboc) 碱基的新型 RNA 亚磷酰胺制备了-保护基团,以及固体支持物。具有正交(光不稳定)2'-O-[(S)-1-(2-硝基苯基)乙氧基]甲基 (=(S)-npeom) 基团的固定化 N6-tboc 保护腺苷。从这些构件中,制备了六聚体寡核糖核苷酸。通过标准下的自动合成。条件。脱离固体支撑物后,得到的完全受保护的序列用 L-苯丙氨酸衍生物氨基酰化。携带光不稳定的N-保护基团。在去除氟化物不稳定的糖和核碱基保护基团后,获得了仍然稳定的、部分用光不稳定基团保护的氨基酰化RNA序列的前体。温和条件下的光解导致
-
FUNCTIONALIZED PHENOLIC COMPOUNDS AND POLYMERS THEREFROM申请人:Bezwada Rao S.公开号:US20090170927A1公开(公告)日:2009-07-02The present invention relates to compounds of formula I, which are functionalized phenolic compounds, and polymers formed from the same. Ar—[O—(X) p —R′] q I Polymers formed from the functionalized phenolics are expected to have controllable degradation profiles, enabling them to release an active component over a desired time range. The polymers are also expected to be useful in a variety of medical applications.
表征谱图
-
氢谱1HNMR
-
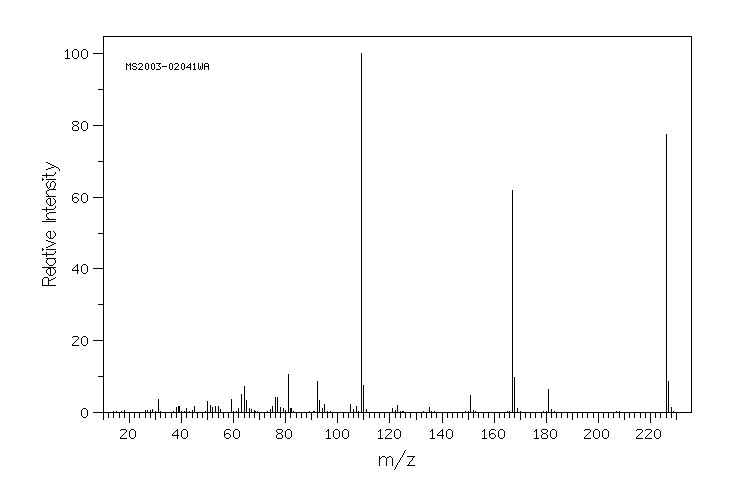
质谱MS
-
碳谱13CNMR
-
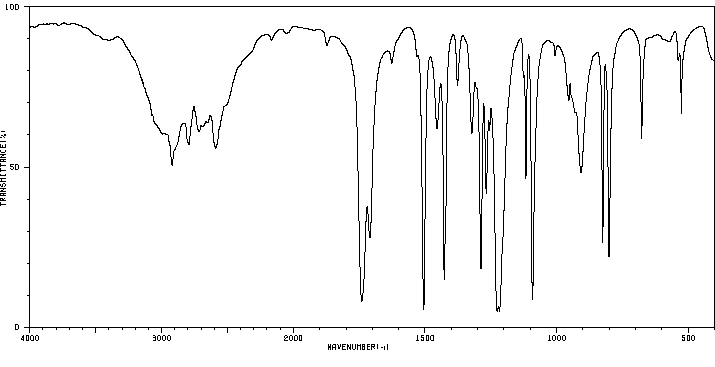
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫