DL-丝氨酸乙酯盐酸盐 | 3940-27-0
中文名称
DL-丝氨酸乙酯盐酸盐
中文别名
2-氨基-3-羟基丙酸乙酯盐酸盐
英文名称
serine ethyl ester hydrochloride
英文别名
DL-serine ethyl ester hydrochloride;Ethyl 2-amino-3-hydroxypropanoate;hydron;chloride;ethyl 2-amino-3-hydroxypropanoate;hydron;chloride
CAS
3940-27-0
化学式
C5H11NO3*ClH
mdl
MFCD00067544
分子量
169.608
InChiKey
JZJQCLZQSHLSFB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:98-100°C
计算性质
-
辛醇/水分配系数(LogP):-1.12
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:72.6
-
氢给体数:3
-
氢受体数:4
安全信息
-
安全说明:S22,S24/25,S26,S36/37/39
-
危险类别码:R22,R36/37/38
-
海关编码:2922509090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存储条件为2-8°C,并需保存在惰性气体中。
SDS
| Name: | Ethyl 2-amino-3-hydroxypropanoate hydrochloride 97% Material Safety Data Sheet |
| Synonym: | DL-Serine ethyl ester hydrochlorid |
| CAS: | 3940-27-0 |
Synonym:DL-Serine ethyl ester hydrochlorid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3940-27-0 | Ethyl 2-amino-3-hydroxypropanoate hydr | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3940-27-0: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 98 - 100 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H12ClNO3
Molecular Weight: 170
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, bases.
Hazardous Decomposition Products:
Hydrogen chloride, chlorine, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3940-27-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 2-amino-3-hydroxypropanoate hydrochloride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 3940-27-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3940-27-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3940-27-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:DL-丝氨酸乙酯盐酸盐 在 2-(3-(4-fluorophenoxy)-3-oxopropyl)benzenediazonium hexafluorophosphate (V) 、 potassium carbonate 作用下, 以 四氢呋喃 、 水 为溶剂, 反应 1.0h, 以75%的产率得到2-diazo-3-hydroxypropanoic acid ethyl ester参考文献:名称:用于将 α-氨基酸衍生物转化为 α-重氮化合物的新型 N-转移试剂摘要:开发了一种新型通用N-转移试剂,可在温和的碱性条件下直接有效地将 α-氨基酮、乙酰胺和酯转化为相应的 α-重氮产物。这种一步合成方法不仅允许从 α-氨基酸衍生物生成 α-取代-α-重氮羰基化合物,而且允许从N-末端二肽制备 α-重氮二肽(32 个例子,高达 91%) .DOI:10.1039/d1cc01285a
-
作为产物:描述:参考文献:名称:LTA4 hydrolase inhibitors摘要:本发明涉及以下公式(1)所示的LTA4水解酶抑制剂组合物。它还涉及它们的治疗,特别是抗炎应用。公开号:US06436973B1
文献信息
-
Efficient Synthesis of New Tetracyclic Benzofuro[3,2-<i>d</i>]-imidazo[1,2-<i>a</i>]pyrimidine-2,5-(1<i>H</i>,3<i>H</i>)-diones作者:Yanggen Hu、Min Liu、Mingwu DingDOI:10.1002/cjoc.201090072日期:2010.2with aromaic isocyanates gave carbodiimides (2), which were allowed to react further with (‐amino ester in the presence of a catalytic amount of sodium ethoxide to give selectively new tetracyclic benzofuro[3,2‐d]imidazo[1,2‐a]pyrimidine‐2,5‐(1H,3H)‐diones (5) in good yields. X‐ray structure analysis of 5i verified the proposed structure and the reaction selectivity.
-
[EN] BICYCLIC COMPOUNDS WHICH INHIBIT BETA-SECRETASE ACTIVITY AND METHODS OF USE THEREOF<br/>[FR] COMPOSES BICYCLIQUES INHIBANT L'ACTIVITE DE LA BETA-SECRETASE ET PROCEDES D'UTILISATION ASSOCIES申请人:ZAPAQ INC公开号:WO2006034277A1公开(公告)日:2006-03-30The present invention provides bicyclic beta-secretase inhibitors and methods for their use, including methods of treating of Alzheimer’s disease.本发明提供了双环β-分泌酶抑制剂及其用途,包括用于治疗阿尔茨海默病的方法。
-
Amino-Containing Compounds Which Inhibit Memapsin 2 Beta-Secretase Activity and Methods of Use Thereof申请人:Ghosh Arun K.公开号:US20080125467A1公开(公告)日:2008-05-29The present invention provides novel beta-secretase inhibitors and methods for their use, including methods of treating of Alzheimer's disease.本发明提供了新型β-分泌酶抑制剂及其使用方法,包括治疗阿尔茨海默病的方法。
-
Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]作者:Ren-Yin Yang、Jing Sun、Ju Xie、Chao-Guo YanDOI:10.1515/hc-2017-0002日期:2017.8.28Abstract Triethylamine-promoted domino cyclodimerization reaction of 3-phenacylideneoxindolines with amino ester hydrochlorides in acetonitrile afforded substituted dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines] in good yields. The relative configuration of the major diastereoisomers was determined by the X-ray diffraction analysis of three single crystal structures.
-
Compounds which inhibit beta-secretase activity and methods of use thereof申请人:Ghosh Arun公开号:US20050239684A1公开(公告)日:2005-10-27The present invention provides novel beta-secretase inhibitors and methods for their use, including methods of treating of Alzheimer's disease.本发明提供了新型β-分泌酶抑制剂及其使用方法,包括治疗阿尔茨海默病的方法。
表征谱图
-
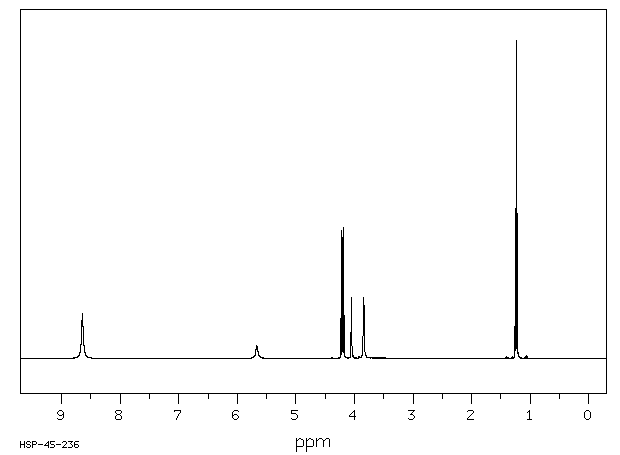
氢谱1HNMR
-
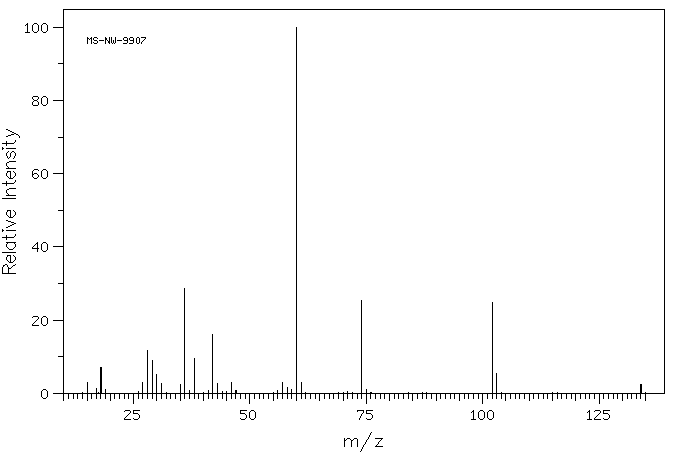
质谱MS
-
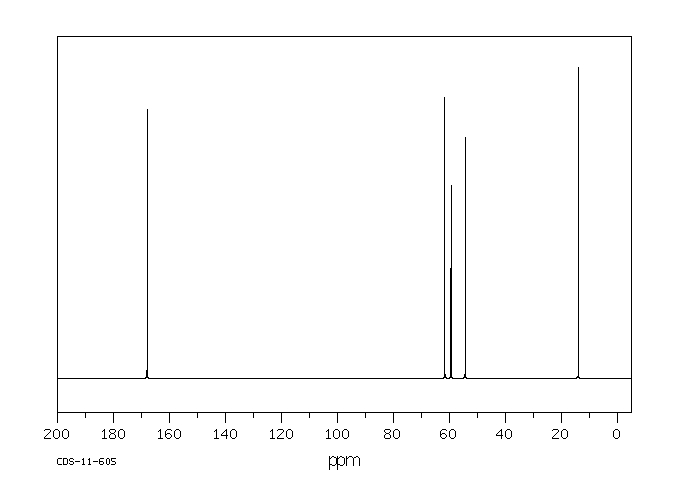
碳谱13CNMR
-
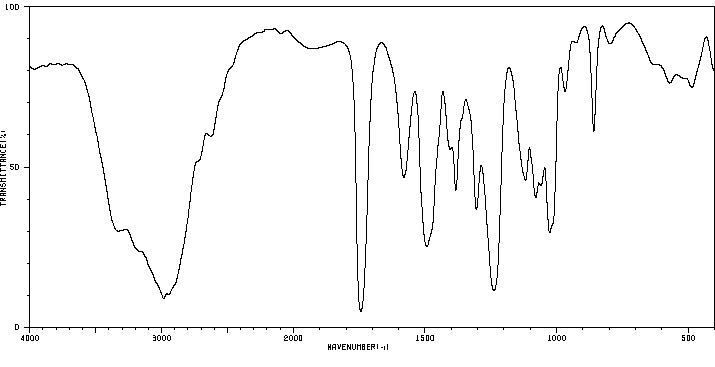
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸