桤木酮 | 33457-62-4
中文名称
桤木酮
中文别名
(4E,6E)-1,7-二苯基-4,6-庚二烯-3-酮
英文名称
alnustone
英文别名
(4E,6E)-1,7-diphenylhepta-4,6-dien-3-one
CAS
33457-62-4
化学式
C19H18O
mdl
——
分子量
262.351
InChiKey
OWMJDOUOHDOUFG-FNCQTZNRSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:60.5-61℃
-
沸点:442.9±34.0 °C(Predicted)
-
密度:1.058±0.06 g/cm3 (20 ºC 760 Torr)
-
溶解度:溶于DMSO和甲醇;
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:20
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
WGK Germany:3
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存储条件:2-8℃,密封保存,并避免光照。
SDS
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:Syntheses and antibacterial activities of 4 linear nonphenolic diarylheptanoids摘要:Four linear nonphenolic diarylheptanoids were synthesized and their antibacterial activities were studied. (S)-2-Me-CBS-catalysed reduction of alnustone with BH3.SMe2 gave (R) (-)(4E,6E)-1,7-diphenylhepta-4,6-dien-3-ol, a natural product. Reduction of alnustone with Na in t-BuOH at -15 degrees C under NH3 atm gave ( E)-1,7-diphenylhept-5-en-3-one as a Birch-type reduction product. t-BuOK catalysed condensation of benzalacetone with propionyl chloride gave (4Z,6E)-5-hydroxy-1,7-diphenylhepta-4,6-dien-3-one, a natural product. (1E,4Z,6E)-5-Hydroxy-4-phenethyl-1,7-diphenylhepta-1,4,6-trien-3-one, a curcuminoid, was synthesized starting from pentan-2,4-dione in 3 steps. The synthesized chemical compounds were applied against 2 gram-positive bacteria (Bacillus cereus and Arthrobacter agilis), 4 gram-negative bacteria (Pseudomonas aeruginosa, Xanthomonas campestris, Klebsiella oxytoca, and Helicobacter pylori), and 1 yeast (Candida albicans) by the disc diffusion method. All of the synthesized compound exhibited different degrees of antimicrobial activity at concentrations between 20-100 mu g/disc against the test organisms.DOI:10.3906/kim-1911-61
-
作为产物:描述:参考文献:名称:GPR52拮抗剂降低亨廷顿病水平并改善亨廷顿舞蹈病相关表型摘要:GPR52是一种孤儿G蛋白偶联受体(GPCR),最近被认为是亨廷顿氏病(HD)(一种无法治愈的单基因神经退行性疾病)的潜在药物靶标。在这项研究中,我们发现GPR52的纹状体敲低可降低成年HdhQ140小鼠的mHTT水平,从而将GPR52确认为HD靶标。此外,通过结构-活性关系(SAR)研究,我们发现了一种高效且特异的GPR52拮抗剂Comp- 43,IC 50值为0.63μM 。进一步的研究表明,Comp- 43通过靶向GPR52降低mHTT水平,并促进了小鼠原代纹状体神经元的存活。此外,体内研究表明Comp- 43不仅降低了mHTT水平,而且还挽救了HdhQ140小鼠的HD相关表型。两者合计,我们的研究证实,抑制GPR52是HD治疗的一种有前途的策略,而GPR52拮抗剂Comp- 43可能充当进一步研究的先导化合物。DOI:10.1021/acs.jmedchem.0c01133
文献信息
-
Synthesis, molecular docking, and antitumoral activity of alnustone-like compounds against estrogen receptor alpha-positive human breast cancer作者:Kaan KÜÇÜKOĞLU、Hatice SEÇİNTİ、Aykut ÖZGÜR、Hasan SEÇEN、Yusuf TUTARDOI:10.3906/kim-1408-72日期:——Alnustone-like compounds are promising inhibitors for estrogen receptor \alpha (ER-\alpha), which is a novel cancer therapeutic target. Therefore, 10 alnustone-like compounds with substituents at the phenyl rings were synthesized by condensation of 4-phenyl-2-butanones and cinnamaldehydes via in situ enamination. The compounds displayed either protective activity or inhibited cell growth and proliferation of human breast cancer cells. Molecular docking studies indicated that the synthesized compounds interact with ER-\alpha efficiently. In this work, the protective and inhibitive roles of the synthesized compounds were related to their functional groups and to their binding mode of action on ER-\alpha protein. The compounds are potential drug candidates as ER-\alpha antagonists.
-
Kuroyanagi, Masanori; Noro, Tadataka; Fukushima, Seigo, Chemical and pharmaceutical bulletin, 1983, vol. 31, # 5, p. 1544 - 1550作者:Kuroyanagi, Masanori、Noro, Tadataka、Fukushima, Seigo、Aiyama, Ritsuo、Ikuta, Akira、et al.DOI:——日期:——
-
VIG, O. P.;BARI, S. S.;SATTAR, M. A.;SHARMA, SANJIV;MAHAJAN, NALINI, J. INDIAN CHEM. SOC., 66,(1989) N, C. 98-100作者:VIG, O. P.、BARI, S. S.、SATTAR, M. A.、SHARMA, SANJIV、MAHAJAN, NALINIDOI:——日期:——
-
KATO, NOBUHARU;HAMADA, YASUMASA;SHIOIRI, TAKAYUKI, CHEM. AND PHARM. BULL., 1984, 32, N 8, 3323-3326作者:KATO, NOBUHARU、HAMADA, YASUMASA、SHIOIRI, TAKAYUKIDOI:——日期:——
-
NMR-Based Metabolic Profiling of <i>Anigozanthos</i> Floral Nectar作者:Dirk Hölscher、Silke Brand、Michael Wenzler、Bernd SchneiderDOI:10.1021/np0705514日期:2008.2.1Nuclear magnetic resonance spectroscopic methods have been used to characterize the chemical composition of floral nectar of Anigozanthos species with a minimum of sample preparation and without derivatization. The nectar of this passerine-pollinated plant is largely dominated by glucose and fructose, while sucrose occurs only at a minor level. Tyrosine, several additional amino acids, and a variety of carboxylic acids were identified and their concentrations estimated. A linear diarylheptanoid was detected as a trace component, marking the first time this type of secondary product in Hemodoraceae has been found.
表征谱图
-
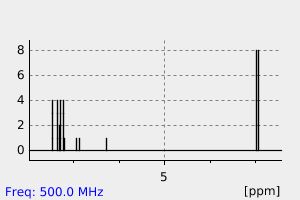
氢谱1HNMR
-
质谱MS
-
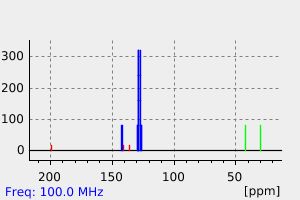
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(11aR)-3,7-双(3,5-二甲基苯基)-10,11,12,13-四氢-5-羟基-5-氧化物-二茚基[7,1-de:1'',7''-fg][1,3,2]二氧杂膦酸
龙血素C
顺-1,7-二苯基-1-庚烯基-5-醇
那洛西芬
赤杨酮
赤杨二醇
血竭素
蒙桑酮C
萘-2,7-二磺基酸,钠盐
苯酚,4-(1,3-二苯基丁基)-2-(1-苯基乙基)-
苯甲酸,2-[[2-[(2-羧基苯基)氨基]-5-(三氟甲基)苯基]氨基]-5-[[[(4-羟基-3-甲氧苯基)甲基]氨基]甲基]-
苯基-[4-(2-苯基乙炔基)苯基]甲酮
苯基-[2-[3-(三氟甲基)苯基]苯基]甲酮
苯基-[2-(2-苯基苯基)苯基]甲酮
苯基-(3-苯基萘-2-基)甲酮
苯基-(2-苯基环己基)甲酮
苯,[(二甲基苯基)甲基]甲基[(甲基苯基)甲基]-
苯,1,3-二[1-甲基-1-[4-(4-硝基苯氧基)苯基]乙基]-
脱甲氧姜黄
紫外吸收剂 234
粗糠柴苦素
硫酸姜黄素
矮紫玉盘素
益智醇
白桦林烯酮;1,7-双(4-羟基苯基)-4-庚烯-3-酮
甲酮,苯基(1,6,7,8-四氢-1-甲基-5-苯基环戊二烯并[g]吲哚-3-基)-
甲酮,[3-(4-甲氧苯基)-1-苯基-9H-芴-4-基]苯基-
甲酮,(4-氯苯基)[1-(4-氯苯基)-3-苯基-9H-芴-4-基]-
环香草酮
溴敌隆
波森
桤木酮
桑根酮D
杨梅醇
杨梅酮
杨梅联苯环庚醇-15-葡糖苷
替拉那韦
替吡法尼(S型对映体)
替吡法尼
曲沃昔芬
姜黄素葡糖苷酸
姜黄素beta-D-葡糖苷酸
姜黄素4,4'-二乙酸酯
姜黄素-d6
姜黄素
姜烯酮 A
奈帕芬胺杂质D
四甲基姜黄素
四氢脱甲氧基二阿魏酰甲烷
四氢姜黄素二乙酸酯