光色素 | 1086-80-2
中文名称
光色素
中文别名
7,8-二甲基咯嗪
英文名称
lumichrome
英文别名
7,8-dimethylalloxazine;7,8-dimethylbenzo[g]pteridine-2,4(1H,3H)-dione;7,8-dimethylisoalloxazine;Riboflavin lumichrome;7,8-dimethyl-1H-benzo[g]pteridine-2,4-dione
CAS
1086-80-2
化学式
C12H10N4O2
mdl
MFCD00005021
分子量
242.237
InChiKey
ZJTJUVIJVLLGSP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:300 °C
-
沸点:385.06°C (rough estimate)
-
密度:1.2532 (rough estimate)
-
溶解度:可溶于水基(轻微)、DMSO(轻微、加热、超声处理)
-
碰撞截面:153.1 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
-
稳定性/保质期:
在常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:18
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:84
-
氢给体数:2
-
氢受体数:4
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2933990090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
7,8-二甲基咯嗪 修改号码:5
模块 1. 化学品
产品名称: 7,8-Dimethylalloxazine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 7,8-二甲基咯嗪
百分比: >95.0%(LC)
CAS编码: 1086-80-2
俗名: Lumichrome
分子式: C12H10N4O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
7,8-二甲基咯嗪 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 浅黄色-黄绿色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
7,8-二甲基咯嗪 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
7,8-二甲基咯嗪 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 7,8-Dimethylalloxazine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 7,8-二甲基咯嗪
百分比: >95.0%(LC)
CAS编码: 1086-80-2
俗名: Lumichrome
分子式: C12H10N4O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
7,8-二甲基咯嗪 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 浅黄色-黄绿色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
7,8-二甲基咯嗪 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
7,8-二甲基咯嗪 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:描述:光色素 在 双氧水 作用下, 以 甲酸 、 水 为溶剂, 反应 2.0h, 生成 Benzo[g]pteridine-2,4(1H,3H)-dione, 7,8-dimethyl-, 10-oxide参考文献:名称:The cybrodins, a new class of sesquiterpenes摘要:报道了一种新的鸟巢真菌Cyathus bulleri产生的一类新的倍半萜化合物“cybrodins”的分离和结构鉴定。Cybrodol(3)、isocybrodol(4)、cybrodal(5)、trisnorcybrodolide(6)和cybrodic acid(7)可以归类为sec-illudalane倍半萜类化合物。提出了导致结构赋值的证据,以及cybrodins之间的化学相关性。讨论了可能的生物合成方式。此外,还分离出了已知的一种新的无萜类倍半萜化合物Pterosin-C(13)和3-甲基琥珀酸(17)。在两次实验中,还获得了一种被认为具有结构21的新的少量倍半萜化合物broderol。DOI:10.1139/v81-310
-
作为产物:描述:参考文献:名称:Flavin-nucleic acid ligand conjugates摘要:该发明涉及黄素-NAL共轭物及其在遗传工程领域中的应用。根据与NAL共轭的黄素的性质,根据本发明的共轭物可以用作(1)在活细胞中特异和高效地切割靶核酸序列的核酸酶,或者(2)用作特异和高效地将NAL(例如反义寡核苷酸)引入细胞内的载体。公开号:EP2422817A1
-
作为试剂:描述:β-cyclocitrylideneacetic acid chloride 在 吡啶 、 光色素 作用下, 以 甲苯 、 乙腈 为溶剂, 反应 40.0h, 生成 tert-butyl (Z)-3-(2,6,6-trimethylcyclohex-1-en-1-yl)acrylate参考文献:名称:β-离子基衍生物的光催化E → Z异构化摘要:据报道,使用廉价的(-)-核黄素(维生素B 2),在402 nm的辐射下,基于视网膜固有的β-离子基,活化二烯的操作简单的E → Z异构化。从光激发的(-)-核黄素到起始E-异构体的选择性能量转移实现了几何异构化。由于与Z-异构体的类似过程效率低下,因此可避免微观可逆性,从而实现定向异构化以生成反热力学产物(产率高达99%,Z / E高达99:1))。谨慎选择光催化剂可使分子间和分子内系统实现化学选择性异构化。从这项研究中建立的原理,以及分子编辑方法,已经促进了基于视网膜支架的截短的三烯的区域选择性异构化的发展。DOI:10.1021/acs.orglett.9b03842
文献信息
-
Flavin–iodine coupled organocatalysis for the aerobic oxidative direct sulfenylation of indoles with thiols under mild conditions作者:Ryoma Ohkado、Tatsuro Ishikawa、Hiroki IidaDOI:10.1039/c8gc00117k日期:——A unique coupled redox organocatalysis system using flavin and iodine catalysts efficiently promoted the metal-free aerobic oxidative direct sulfenylation of indoles with thiols at ambient temperature without any sacrificial reagents, except environmentally benign molecular oxygen. Biomimetic flavin catalysis plays multiple roles in aerobic oxidative transformations, not only regenerating I2 from in
-
Comparison of riboflavin-derived flavinium salts applied to catalytic H<sub>2</sub>O<sub>2</sub>oxidations作者:Takuya Sakai、Takuma Kumoi、Tatsuro Ishikawa、Takahiro Nitta、Hiroki IidaDOI:10.1039/c8ob00856f日期:——properties, and their catalytic activity in H2O2 oxidations of sulfide, tertiary amine, and cyclobutanone. Reflecting the difference between the π-conjugated ring structures, the flavinium salts displayed very different redox properties, with reduction potentials in the order of: 5-ethylisoalloxazinium > 5-ethylalloxazinium > 1,10-ethylene-bridged alloxazinium. A comparison of their catalytic activity revealed从市场上可买到的核黄素制备了一系列黄酮盐,5-乙基异all杂嗪鎓,5-乙基ethyl杂嗪鎓和1,10-乙烯桥联的四氧嘧啶鎓三氟甲磺酸盐。这项研究提出了它们的光学和氧化还原特性,以及它们在H 2 O 2中的催化活性之间的比较。硫化物,叔胺和环丁酮的氧化。反映了π-共轭环结构之间的差异,黄酮盐显示出非常不同的氧化还原性质,其还原电位的顺序为:5-乙基异四氮杂鎓> 5-乙基四氧杂鎓> 1,10-乙烯桥联的四氧杂嗪鎓。比较它们的催化活性表明,三氟甲磺酸5-乙基异恶唑嗪能特异性氧化硫化物和环丁酮,而三氟甲磺酸5-乙基异恶唑嗪能平稳地氧化叔胺。1,10-桥联的三氟甲磺酸铝恶唑鎓盐,可以很容易地从核黄素中大量获得,对硫化物和环丁酮的H 2 O 2氧化表现出中等的催化活性。
-
Synthesis of Riboflavines, Quinoxalinones and Benzodiazepines through Chemoselective Flow Based Hydrogenations作者:Marcus Baumann、Ian Baxendale、Christian Hornung、Steven Ley、Maria Rojo、Kimberley RoperDOI:10.3390/molecules19079736日期:——Robust chemical routes towards valuable bioactive entities such as riboflavines, quinoxalinones and benzodiazepines are described. These make use of modern flow hydrogenation protocols enabling the chemoselective reduction of nitro group containing building blocks in order to rapidly generate the desired amine intermediates in situ. In order to exploit the benefits of continuous processing the individual steps were transformed into a telescoped flow process delivering selected benzodiazepine products on scales of 50 mmol and 120 mmol respectively.
-
Mechanochemical <i>N</i>-alkylation of imides作者:Anamarija Briš、Mateja Đud、Davor MargetićDOI:10.3762/bjoc.13.169日期:——The mechanochemical N-alkylation of imide derivatives was studied. Reactions under solvent-free conditions in a ball mill gave good yields and could be put in place of the classical solution conditions. The method is general and can be applied to various imides and alkyl halides. Phthalimides prepared under ball milling conditions were used in a mechanochemical Gabriel synthesis of amines by their
-
Turning a riboflavin-binding protein into a self-sufficient monooxygenase by cofactor redesign作者:Gonzalo de Gonzalo、Christian Smit、Jianfeng Jin、Adriaan J. Minnaard、Marco W. FraaijeDOI:10.1039/c1cc14039f日期:——By cofactor redesign, self-sufficient monooxygenases could be prepared. Tight binding of N-alkylated flavins to riboflavin-binding protein results in the creation of artificial flavoenzymes capable of H2O2-driven enantioselective sulfoxidations. By altering the flavin structure, opposite enantioselectivities could be achieved, in accordance with the binding mode predicted by in silicoflavin-protein docking of the unnatural flavin cofactors. The study shows that cofactor redesign is a viable approach to create artificial flavoenzymes with unprecedented activities.
表征谱图
-
氢谱1HNMR
-
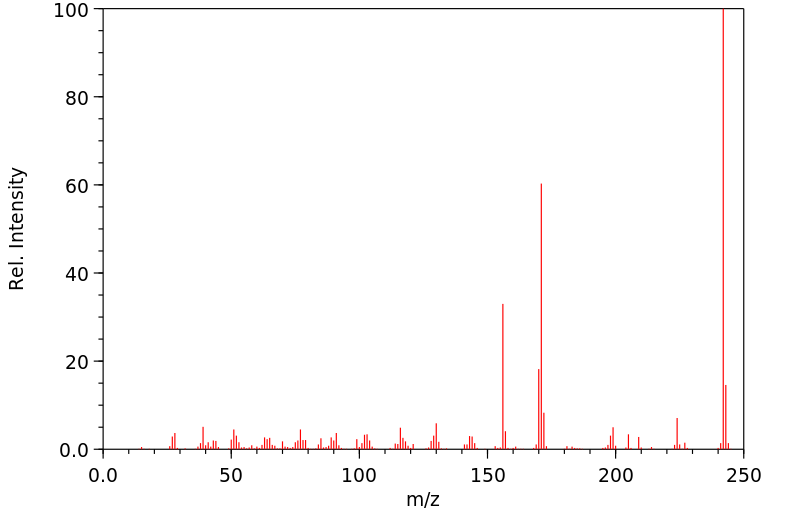
质谱MS
-
碳谱13CNMR
-
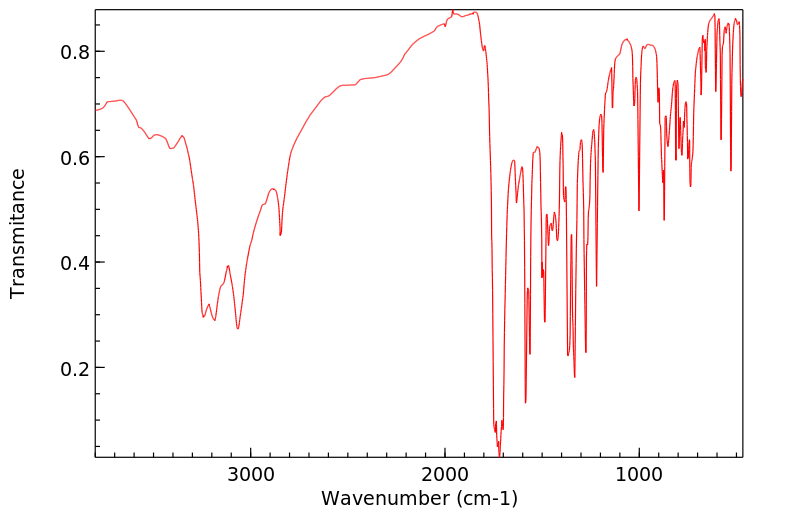
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄素酰色氨酸
高蝶酸
骏河毒素
酵母粉
诺米林酸17-β-D-吡喃葡萄糖苷
蝶酸
蝶啶3-氧化物
蝶啶-6-基-甲醇
蝶啶-4,6-二胺
蝶啶-2,4-二胺
蝶呤-6-羧酸
苯癸酸,2-羟基-3,4-二甲氧基-6-甲基
苯并[g]蝶啶-4a(2H)-基,5-乙基-3,4,5,10-四氢-3,7,8,10-四甲基-2,4-二羰基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,5-乙酰基-5,10-二氢-1,3-二甲基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,5,10-二氢-7,8-二甲基-
苯并[g]蝶啶-2,4(1H,3H)-二酮,1,7,8-三甲基-
羧甲基黄素
羟基-2-吡啶酮
维生素 B2
维他命 B2
硫酸氢3-(6,7-二氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N-乙基-N-(2-羟基乙基)丙烷-1-铵
硫酸氢2-(7,8-二氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N,N-二甲基乙铵
甲氨蝶呤钠
甲氨蝶呤杂质1
生物蝶呤-d3
生物喋呤中间体
环己烯,3-氟-4-(甲硫基)-,反-(9CI)
玫瑰黄色素
溴化氢溴化1-(2-氨基乙基)-3-甲基-4-[(Z)-2-萘-1-基乙烯基]吡啶正离子
氯化3-(7-氯-2,4-二羰基-3,4-二氢苯并[g]蝶啶-10(2H)-基)-N,N-二甲基丙烷-1-铵
氨蝶呤钠
氨苯蝶啶
氨甲酸,[(1S)-2-羟基-1-甲基丙基]-,1,1-二甲基乙基酯(9CI)
氨甲蝶呤
氨基蝶呤
核黄素还原
核黄素杂质Q
核黄素5'-硫酸盐
核黄素3′,4′-二磷酸酯
核黄素-4'-磷酸
核黄素-3'-磷酸盐
核黄素,2',3',4',5'-四乙酸酯
核黄素 5'-丁酸酯
核黄素
无色喋呤
异黄蝶呤
己二酸,2-[[4-[[(2-氨基-1,4,5,6,7,8-六氢-4-羰基-6-蝶啶基)甲基]氨基]苯甲酰]氨基]-
左亚叶酸钙杂质
左亚叶酸钙
四氢蝶酰五谷氨酸酯