2,2,5,6-四甲基-4H-1,3-二氧杂-4-酮 | 87769-39-9
中文名称
2,2,5,6-四甲基-4H-1,3-二氧杂-4-酮
中文别名
2,2,5,6-四甲基-4H-1,3-二氧杂-4-己酮
英文名称
2,2,5,6-tetramethyl-1,3-dioxin-4-one
英文别名
2,2,5,6-tetramethyl-4H-1,3-dioxin-4-one
CAS
87769-39-9
化学式
C8H12O3
mdl
MFCD00060139
分子量
156.181
InChiKey
NZEPEQVPVAXLSY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:72°C 0,3mm
-
密度:1,07 g/cm3
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.625
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2932999099
-
储存条件:存储条件为0-10°C,并需置于惰性气体中,避免与空气接触和加热。
SDS
2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
None
Pictograms or hazard symbols
Signal word Warning
Combustible liquid
Hazard statements
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wear protective gloves and eye/face protection.
Store in a well-ventilated place. Keep cool.
[Storage]
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
>95.0%(GC)
Percent:
CAS Number: 87769-39-9
Chemical Formula: C8H12O3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Rinse cautiously with water for several minutes. Remove contact lenses, if present
Eye contact:
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Very pale yellow - Yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 72°C/0.4kPa
Flash point: No data available
2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 1.07
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Open flame
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
None
Pictograms or hazard symbols
Signal word Warning
Combustible liquid
Hazard statements
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wear protective gloves and eye/face protection.
Store in a well-ventilated place. Keep cool.
[Storage]
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
>95.0%(GC)
Percent:
CAS Number: 87769-39-9
Chemical Formula: C8H12O3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Rinse cautiously with water for several minutes. Remove contact lenses, if present
Eye contact:
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Very pale yellow - Yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 72°C/0.4kPa
Flash point: No data available
2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 1.07
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Open flame
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,2,5,6-Tetramethyl-4H-1,3-dioxin-4-one
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:2,2,5,6-四甲基-4H-1,3-二氧杂-4-酮 以 乙醇 为溶剂, 以40%的产率得到2-甲基乙酰乙酸乙酯参考文献:名称:Sato, Masayuki; Ogasawara, Hiromichi; Takayama, Kazuhisa, Heterocycles, 1987, vol. 26, # 10, p. 2611 - 2614摘要:DOI:
-
作为产物:描述:参考文献:名称:Sato, Masayuki; Ogasawara, Hiromichi; Oi, Keiichi, Chemical and pharmaceutical bulletin, 1983, vol. 31, # 6, p. 1896 - 1901摘要:DOI:
-
作为试剂:描述:2-甲基-3-氧代丁酸叔丁酯 在 4-乙酰氨基苯磺酰叠氮 、 2,2,5,6-四甲基-4H-1,3-二氧杂-4-酮 作用下, 以 乙腈 为溶剂, 反应 0.5h, 以27%的产率得到t-butyl 2-diazopropanoate参考文献:名称:Ru(II)-Pheox催化的Si-H插入反应:对映体富集的碳和硅中心的构建摘要:我们建立了一个高度对映选择性的Si-H插入反应,使用Ru(II)-Pheox催化剂在碳和硅原子上构建了手性中心。[小α]-甲基-[小α]-重氮酯的催化不对称Si-H插入反应进行...DOI:10.1039/c7cc01070b
文献信息
-
Chemoenzymatic Synthesis of Acyl Coenzyme A Substrates Enables <i>in Situ</i> Labeling of Small Molecules and Proteins作者:Vinayak Agarwal、Stefan Diethelm、Lauren Ray、Neha Garg、Takayoshi Awakawa、Pieter C. Dorrestein、Bradley S. MooreDOI:10.1021/acs.orglett.5b02113日期:2015.9.18generate fully functional acyl coenzyme A molecules that are then used as substrates to drive in situ acyl transfer reactions is described. Mass spectrometry based assays to verify the identity of acyl coenzyme A enzymatic products are also illustrated. The approach is responsive to a diverse array of carboxylic acids that can be elaborated to their corresponding coenzyme A thioesters, with potential applications
-
Sythesis of 1,3-dioxin-4-ones and their use in synthesis. XVIII. Synthesis of azetidin-2-ones from 1,3-dioxin-4-ones via 3-hydroxycarboxamides.作者:Jun-ichi SAKAKI、Satoshi KOBAYASHI、Masayuki SATO、Chikasra KANEKODOI:10.1248/cpb.37.2952日期:——A general method for the synthesis of azetidin-2-ones from 1, 3-dioxin-4-ones is described. The method consists of 1) the formatin of β-ketocarboxamides, 2) their reduction to 3-hydroxycarboxamides, 3) mesylationg, and 4) base-mediated cyclization of 3-mesyloxycarboxamides to the final azetidinones. Stereochemical demand in the cyclizatino step has been clarified by using 5, 6-tri- and -tetramethylene derivatives of 2, 2-dimethyl-1, 3-dioxin-4-one. Microbiological reduction of the acetoacetamides by baker's yeast gave (S)-3-hydroxybutanamides of ≥98% optical purity, whose cyclization afforded (R)-4-methylazetidin-2-ones.
-
Photochemical ring-contraction of fused bicyclic 4-pyrones: A novel 2-step cyclopentannulation approach作者:F.G. West、P.V. Fisher、G.U. Gunawardena、Scott MitchellDOI:10.1016/s0040-4039(00)60630-5日期:1993.9Fused bicyclic 4-pyrones were prepared by condensation of enamines derived from cyclic ketones with diketene or substituted 1,3-dioxin-4-ones. Photolysis in a hydroxylic medium led to bicyclo[n3.0]alkenones bearing oxygenation at both angular positions. This process is occurs via regioselective nucleophilic solvent attack on the intermediate tricyclic oxyallyl zwitterion. The efficiency of the transformation
-
Synthesis of (+)-7,20-Diisocyanoadociane and Liver-Stage Antiplasmodial Activity of the Isocyanoterpene Class作者:Hai-Hua Lu、Sergey V. Pronin、Yevgeniya Antonova-Koch、Stephan Meister、Elizabeth A. Winzeler、Ryan A. ShenviDOI:10.1021/jacs.6b03899日期:2016.6.15marine metabolite with potent antimalarial activity, was synthesized as a single enantiomer in 13 steps from simple building blocks (17 linear steps). Chemical synthesis enabled identification of isocyanoterpene antiplasmodial activity against liver-stage parasites, which suggested that inhibition of heme detoxification does not exclusively underlie the mechanism of action of this class.
-
Synthesis of 1,3-dioxin-4-ones and their use in synthesis. Part XXIV. A novel synthesis of .BETA.-ketothioesters.作者:Jun-ichi SAKAKI、Satoshi KOBAYASHI、Masayuki SATO、Chikara KANEKODOI:10.1248/cpb.38.2262日期:——A new general one-pot method for high-yield synthesis of S-alkyl β-ketothioesters by heating of 6-mono- and 5, 6-disubstituted 1, 3-dioxin-4-ones and thiols in an appropriate aprotic solvent is described. This procedure is available for large-scale preparation of a variety of β-ketothioesters.
表征谱图
-
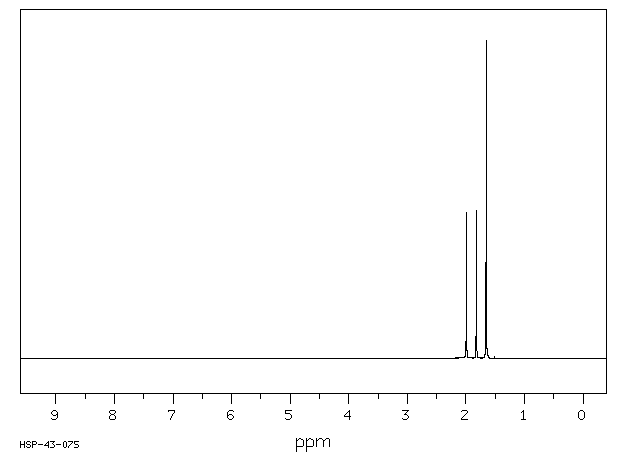
氢谱1HNMR
-
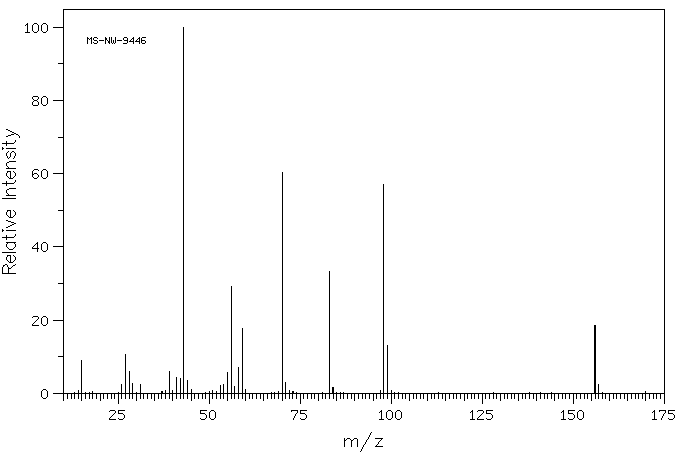
质谱MS
-
碳谱13CNMR
-
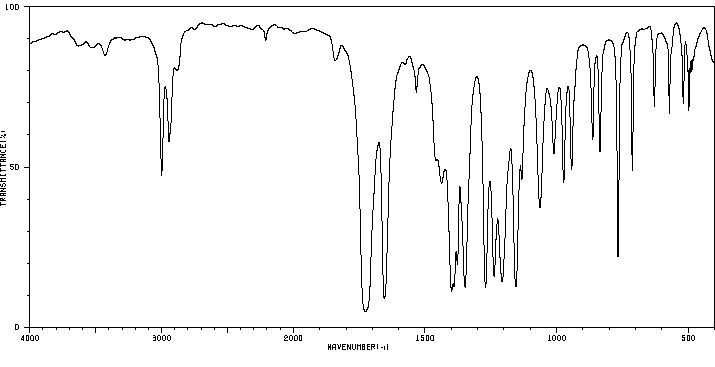
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷