柠檬酸三乙酯 | 77-93-0
中文名称
柠檬酸三乙酯
中文别名
柠檬酸乙酯;三乙基檸檬酸;2-羟基-1,2,3-丙三羧酸三乙酯;TEC
英文名称
citric acid triethyl ester
英文别名
triethyl citrate;triethyl 2-hydroxypropane-1,2,3-tricarboxylate
CAS
77-93-0
化学式
C12H20O7
mdl
MFCD00009201
分子量
276.287
InChiKey
DOOTYTYQINUNNV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-55 °C
-
沸点:235 °C/150 mmHg (lit.)
-
密度:1.14 g/mL at 25 °C (lit.)
-
蒸气密度:9.7 (vs air)
-
闪点:>230 °F
-
溶解度:在水中可溶
-
LogP:1.267 (est)
-
物理描述:Liquid
-
颜色/状态:Oily liquid
-
气味:Fruity odor
-
味道:Bitter taste
-
蒸汽压力:6.87X10-4 mm Hg at 25 °C (extrapolated)
-
稳定性/保质期:
-
分解:When heated to decomposition it emits acrid smoke and irritatiing fumes.
-
粘度:35.2 cP at 25 °C
-
折光率:Index of refraction = 1.4455 at 20 °C/D
-
保留指数:1655;1627;1655
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:19
-
可旋转键数:11
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:99.1
-
氢给体数:1
-
氢受体数:7
ADMET
代谢
Triethyl citrate is hydrolyzed in vivo to citric acid and ethanol. Triethyl citrate appeared to be hydrolyzed at a slower rate with human serum compared to rat serum.
来源:Hazardous Substances Data Bank (HSDB)
代谢
新鲜收集的大鼠或人血清样本中加入了三乙基柠檬酸盐,并测量了4小时内三乙基柠檬酸盐的消失情况。大鼠血清迅速水解三乙基柠檬酸盐(15分钟内),但在人血清中水解速率要慢得多,并且在4小时测试期结束时仍未完全水解。
Samples of freshly collected rat or human serum were spiked with triethyl citrate and the disappearance of the triethyl citrate measured over a 4 hr period. Triethyl citrate was rapidly hydrolysed by rat serum (15 min), but hydrolysisoccurred at a much slower rate in human serum and was not complete at the end of the 4 hr test period.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Rat-, mouse- and human-liver homogenates as well as serum enzymes hydrolyse triethyl citrate to 1 mol citric acid and 3 mol ethanol/mol ester.
来源:Hazardous Substances Data Bank (HSDB)
代谢
三乙基柠檬酸酯预计会被酯酶和细胞色素P450酶广泛代谢,并在β-氧化或柠檬酸循环中分解,或者在后续经过葡萄糖醛酸化。该物质假定以代谢物(即与葡萄糖醛酸的共轭物)的形式通过尿液排出,在一定程度上也通过胆汁排出(如果在β-氧化和柠檬酸循环中没有完全代谢)。
/Triethyl citrate/ is expected to be extensively metabolized by esterases and cytochrome P450 enzymes and break-down in the beta-oxidation or citric acid cycle or in cases subsequent glucuronidation. The substance is assumed to be excreted (if not metabolized completely in beta-oxidation and citric cycle) as metabolites (i.e. conjugates with glucuronic acid) via urine and to a lower extent via bile.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:三乙基柠檬酸是一种无色、油性液体,具有苦味。它用作硝化纤维素和天然树脂的溶剂和塑化剂,软化剂,油漆去除剂,粘合剂,香水基料,食品添加剂(不超过0.25%)作为乳化剂和作为风味保持剂。人类暴露和毒性:在人类研究中,含有20%三乙基柠檬酸的凡士林不是原发性刺激物或致敏物。动物研究:在豚鼠最大化测试中,未稀释的三乙基柠檬酸在表皮诱导期间是一种强烈的致敏剂。33.3%的三乙基柠檬酸在兔眼内产生刺激性。将100 mg/kg bw剂量的三乙基柠檬酸静脉注射给兔子,会使活动能力和呼吸明显增加。一组20只小鼠每天腹膜内注射350 mg/kg bw的三乙基柠檬酸,持续14天,其平均生长速率略低于对照组动物。两组在红细胞和白细胞计数、凝血时间和血红蛋白水平方面没有差异。对两只动物在尸检时检查肝脏、肺和肾脏组织,没有发现病理性细胞变化。在0.5至10 mg/kg b.w.的剂量范围内,三乙基柠檬酸对鸡胚胎不具有致畸性。使用三乙基柠檬酸(0.4%-1.6%)对鼠伤寒沙门氏菌TA1535、TA1537、TA1538进行的Ames试验,在有无代谢激活的情况下均为阴性。
IDENTIFICATION AND USE: Triethyl citrate is a colorless, oily liquid with bitter taste. It is used as solvent and plasticizer for nitrocellulose and natural resins, softener, paint removers, agglutinant, perfume base, food additive (not over 0.25%) as an emulsifier and as a flavor-preserving agent. HUMAN EXPOSURE AND TOXICITY: Triethyl citrate 20% in petrolatum was not a primary irritant or sensitizer in human studies. ANIMAL STUDIES: Triethyl citrate, applied undiluted during epidermal induction, was a strong sensitizer in a guinea pig maximization test. Triethyl citrate 33.3% produced irritation in rabbit eyes. Intravenous administration of a 100 mg/kg bw dose of triethyl citrate to rabbits produced a marked increase in motor activity and respiration. A group of 20 mice given intraperitoneal doses of 350 mg/kg bw of triethyl citrate daily for 14 days had a slightly lower mean growth rate than control animals. No differences were seen in the two groups in erythrocyte and leucocyte blood cell count, clotting time and hemoglobin levels. Examination of the liver, lung and kidney tissues of two animals at necropsy revealed no pathological cellular changes. At doses ranging from 0.5 to 10 mg/kg b.w. triethyl citrate was nonteratogenic in the chicken embryo. Ames test using triethyl citrate (0.4%-1.6%) on Salmonella typhimurium TA1535, TA1537, TA1538 was negative with and without metabolic activation.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
对人类无致癌性(未列入国际癌症研究机构IARC清单)。
No indication of carcinogenicity to humans (not listed by IARC).
来源:Toxin and Toxin Target Database (T3DB)
毒理性
该物质可以通过吸入其气溶胶和通过吞食被吸收进人体。
The substance can be absorbed into the body by inhalation of its aerosol and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
咳嗽。
Cough.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
红色。
Redness.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
为了评估三乙基柠檬酸盐的毒理学行为,已对现有实验和预测的物理化学数据进行了评估。预计该物质会被很好地吸收。感兴趣的物质的任何代谢物的吸收都是快速且完全的。关于通过吸入暴露后的吸收,由于该化学物质具有较低的蒸气压,很明显,吸入后该物质的可用性较差。鉴于其亲脂性(LogPow 1.17) - 如果被吸收 - 预计它将直接通过呼吸道上皮被吸收。由于分子量和LogPow,预计该物质在通过皮肤暴露后也会较差地被吸收到角质层和一定程度上到表皮。此外,预计通过皮肤的系统性毒性较低,这一点已通过三乙基柠檬酸盐的急性皮肤研究的结果得到证实,该研究中获得了5000 mg/kg bw的LD50值。关于在体内的分布,预计三乙基柠檬酸盐主要存在于循环系统中(由于其水溶性)。实验确定的LogPow值、水溶性以及预测的关于三乙基柠檬酸盐吸收的行为,并不表明该物质有积累的潜力。
In order to assess the toxicological behavior of triethyl citrate, the available experimental and predicted physico-chemical data have been evaluated. The substance is expected to be absorbed very well. The absorption of any metabolite of the substances of interest is fast and complete. Concerning the absorption after exposure via inhalation, as the chemical has a low vapor pressure, it is clear, that the substance is poorly available after inhalation. Given its lipophilicity (LogPow 1.17) - if absorbed - it is expected to be absorbed directly across the respiratory tract epithelium. The substance is expected to be also poorly absorbed following dermal exposure into the stratum corneum and to a certain extent into the epidermis, due to its molecular weight and its LogPow. In addition, the systemic toxicity via the skin is assumed to be low and this has been proven with the results of the acute dermal study with triethyl citrate, in which a LD50 of 5000 mg/kg bw has been obtained. Concerning the distribution in the body, triethyl citrate is expected to be mainly available in the circulatory system (due to its water solubility). The experimentally determined LogPow value, the water solubility and predicted behavior concerning absorption of the substance triethyl citrate do not indicate a potential for accumulation.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R20
-
WGK Germany:1
-
海关编码:2918150000
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:GE8050000
-
储存条件:该产品应密封存放于阴凉干燥处。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 柠檬酸三乙酯;2-羟基-1,2,3-丙烷三羧酸三乙酯 |
| 化学品英文名称: | Triethyl citrate |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 77-93-0 |
| 分子式: | C 12 H 20 O 7 |
| 分子量: | 276.32 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:柠檬酸三乙酯;2-羟基-1,2,3-丙烷三羧酸三乙酯 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 据实验资料,对眼睛、皮肤无刺激作用。目前,未见中毒报告。 |
| 环境危害: | |
| 燃爆危险: |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或氧化剂,有引起燃烧的危险。 |
| 有害燃烧产物: | |
| 灭火方法及灭火剂: | 雾状水、干粉、砂土、二氧化碳。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 150.6 |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 用水冲洗,经稀释的污水放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | |
| 储存注意事项: |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 提供良好的自然通风条件。 |
| 呼吸系统防护: | 一般不需特殊防护。 |
| 眼睛防护: | 一般不需特殊防护。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 防护要求不高。 |
| 其他防护: | 工作后,淋浴更衣。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色油状液体,有果香,味苦。 |
| pH: | |
| 熔点(℃): | -55 |
| 沸点(℃): | 294 |
| 相对密度(水=1): | 1.1369(20℃) |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | 0.133/107℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 150.6 |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 12 H 20 O 7 |
| 分子量: | 276.32 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于水,可混溶于乙醇、乙醚,溶于酯。 |
| 主要用途: | 用作聚氯乙烯的增塑剂,也用于制药物、香料。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:7000mg/kg(大鼠经口) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。保持容器密封。应与氧化剂分开存放。搬运时要轻装轻卸,防止包装及容器损坏。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 6 |
| MSDS修改日期: | 年月日 |
制备方法与用途
理化性质
柠檬酸三乙酯在常温常压下为无色油性液体,在25℃时于水中的溶解度为6.5g/100cm³。这种化合物具有甜而愉快的果子酒和梅子般的香气,并且能溶于大多数有机溶剂,但难溶于油。它与许多纤维素、聚氯乙烯、聚醋酸乙烯树脂及氯化橡胶等材料有良好的相容性。
含量分析精确称取试样1.5g,放入500ml标准口烧瓶中,加入异丙醇25ml和水25ml。吸取0.5mol/L氢氧化钠50mL,移入混合液内,加若干沸石片,连接上水冷却冷凝器,并回流1.5小时后冷却,用约20ml的水冲洗冷凝器,加入5滴溴百里酚蓝试液(T5-56),使用0.5mol/L硫酸滴定过量的碱。进行空白试验,每mL 0.5mol/L氢氧化钠相当于柠檬酸三乙酯(C₁₇H₂₀O₇)46.05mg。
毒性- ADI:0~20mg/kg(FAO/WHO,2001)
- GRAS(FDA,§182.1911,2000)
- LD₅₀:5900 mg/kg(大鼠,经口)
- FEMA(mg/kg):软饮料 13,冷饮 47,糖果 180,焙烤食品 230,布丁类 10。
- FDA,§182.1911(1994):限用于干蛋白,GMP为限。
- 添加剂中文名称:柠檬酸三乙酯
- 允许使用该种添加剂的食品中文名称:食品
- 添加剂功能:食品用香料
- 最大允许使用量(g/kg):用于配制香精的各香料成分不得超过在GB 2760中的最大允许使用量和最大允许残留量
柠檬酸三乙酯为无色透明液体,稍有气味,在25℃时于水中的溶解度为6.5g/100cm³。这种化合物能溶于大多数有机溶剂,但难溶于油类,并与许多纤维素、聚氯乙烯、聚醋酸乙烯树脂及氯化橡胶等材料有良好的相容性。
用途柠檬酸三乙酯是一种无毒增塑剂,广泛应用于纤维素树脂和乙烯基树脂的增塑剂。在食品中作为膨松保型剂能很好地提高烘烤食品的发泡性能,改善膨松状态;作为抗氧剂稳定大豆油、色拉油、人造奶油、起酥油及其他食用油脂;作为增香剂可用于软饮料冷饮、糖果、焙烤食品中增加风味。还被用作螯合剂和载体溶剂。特别适用于油墨涂料、无毒PVC造粒、制药工业、儿童软质玩具、医用制品、调配香精香料及化妆品制造等行业。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙酰柠檬酸三乙酯 triethyl O-acetylcitrate 77-89-4 C14H22O8 318.324 乙酰柠檬酸三丁酯 acetyltributyl citrate 77-90-7 C20H34O8 402.485 —— ((S)-4-Methoxycarbonylmethyl-5-oxo-[1,3]dioxolan-4-yl)-acetic acid 199341-50-9 C8H10O7 218.163 —— 5,5-Bis-methoxycarbonylmethyl-[1,3]dioxolan-4-on 77862-72-7 C9H12O7 232.19 柠檬酸 citric acid 77-92-9 C6H8O7 192.125 5-氧代-1,3-二氧戊环-4,4-二乙酸 5-oxo-1,3-dioxolan-4-ylidenedi(acetic acid) 144-16-1 C7H8O7 204.136 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2-diethyl-3-methyl-2-hydroxypropane-1,2,3-tricarboxylate 1242516-58-0 C11H18O7 262.26 2-羟基-1,2,3-丙烷三羧酸二乙酯 1,3-diethyl citrate 32074-56-9 C10H16O7 248.233 柠檬酸三戊基脂 tripentyl citrate 70289-34-8 C21H38O7 402.529 —— Tris(2-hydroxyethyl) 2-hydroxypropane-1,2,3-tricarboxylate 86714-13-8 C12H20O10 324.285 3-(乙氧羰基)-3-羟基戊二酸 citric acid β-ethyl ester 4552-01-6 C8H12O7 220.179 柠檬酸三甲酯 trimethyl citrate 1587-20-8 C9H14O7 234.206 柠檬酸单乙酯 ethyl citrate 30306-93-5 C8H12O7 220.179 —— 1,5-diethyl citrate 101996-63-8 C10H16O7 248.233 2-乙酰柠檬酸三乙酯 triethyl O-acetylcitrate 77-89-4 C14H22O8 318.324 柠檬酸 citric acid 77-92-9 C6H8O7 192.125 —— triethyl 2-benzyloxy-1,2,3-propanetricarboxylate 112031-19-3 C19H26O7 366.411 —— Triethyl 2-(2-methylprop-2-enoyloxy)propane-1,2,3-tricarboxylate 186351-92-8 C16H24O8 344.362 —— triethyl propane-1,2,3-tricarboxylate 1188-22-3 C12H20O6 260.287 —— 3-ethoxycarbonyl-3-phosphonooxypentanedioic acid diethyl ester 73319-80-9 C12H21O10P 356.266 —— 3-ethoxycarbonyl-3-(hydroxymethylphosphoryloxy)pentanedioic acid diethyl ester 929042-26-2 C13H23O10P 370.293 —— 3-ethoxycarbonyl-3-(ethoxymethoxyphosphoryloxy)pentanedioic acid diethyl ester 929042-27-3 C15H27O10P 398.347 —— Triethyl 2-benzoyloxypropane-1,2,3-tricarboxylate 145867-24-9 C19H24O8 380.395 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Wolfrum; Pinnow, Journal fur praktische Chemie (Leipzig 1954), 1918, vol. <2>97, p. 26,33摘要:DOI:
-
作为产物:描述:5-氧代-1,3-二氧戊环-4,4-二乙酸 在 甲醇 、 sodium hydroxide 、 pig liver esterase 、 丙醛二乙基乙缩醛 、 DOWEX 50WX4 、 phosphate buffer pH=7 、 三乙胺 作用下, 反应 26.0h, 生成 柠檬酸三乙酯参考文献:名称:Regio- and enantioselectivity of the enzyme-catalyzed hydrolysis of citric acid derivatives摘要:The hydrolysis of triethyl citrate in the presence of three serine proteases (chymotrypsin, subtilisin in BPN', Is subtilisin carlsberg) is highly regioselective and gives the symmetric diester. Several lipases and proteases have the complementary regioselectivity and give the chiral diester. Pig liver esterase, Aspergillus niger lipase and Candida antarctica lipase give the chiral (R)-diester with good regio- and enantioselectivity. The stereoselective hydrolysis of the meso citric derivatives 7a,b in the presence of Candida antarctica lipase gives the corresponding (R)-monoester. (C) 1998 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0957-4166(98)00448-0
-
作为试剂:参考文献:名称:JP5682397摘要:公开号:
文献信息
-
ACYL-HYDRAZONE AND OXADIAZOLE COMPOUNDS, PHARMACEUTICAL COMPOSITIONS CONTAINING THE SAME AND USES THEREOF申请人:Universidade Federal de Santa Catarina公开号:US20150191445A1公开(公告)日:2015-07-09The present invention relates to acyl-hydrazone compounds, in particular 3,4,5-trimethoxyphenyl-hydrazide derivatives, as well as the oxadiazole analogs thereof and other similar compounds, and to the pharmaceutical use of the same for the treatment of various diseases associated with cell proliferation, such as leukemias, including acute lymphoblastic leukemia (ALL), tumours and inflammation. Acyl-hydrazones have been obtained having activity similar to that of the compound used as a standard in experiments (colchicine). The greater selectivity of the compounds according to the invention is an important feature, associated with fewer side effects than the pharmaceuticals used at present in clinical treatments. The synthetised acyl-hydrazones, more particularly the compounds 02 and 07, exhibited important antileukemic activity, which suggests 02 and 07 as candidates to pharmaceutical prototypes, or to pharmaceuticals for the treatment of leukemias, in particular acute lymphoblastic leukemia (ALL), tumours and other proliferative diseases, such as inflammation. The action mechanism of the most active compounds was determined by using DNA microarrays and subsequent tests indicated by the chip, besides selectivity studies in healthy human lymphocytes.本发明涉及酰基腙化合物,特别是3,4,5-三甲氧基苯基腙衍生物,以及其噁二唑类似物和其他类似化合物,以及它们在治疗与细胞增殖相关的各种疾病,如白血病(包括急性淋巴细胞白血病(ALL))、肿瘤和炎症方面的药用。已获得具有与实验中使用的化合物(秋水仙碱)相似活性的酰基腙。根据本发明的化合物具有更大的选择性,与目前在临床治疗中使用的药物相比,副作用更少是一个重要特征。合成的酰基腙,尤其是化合物02和07,表现出重要的抗白血病活性,这表明02和07可能成为药物原型的候选,或用于治疗白血病,特别是急性淋巴细胞白血病(ALL)、肿瘤和其他增殖性疾病,如炎症的药物。最活性化合物的作用机制是通过使用DNA微阵列确定的,并且通过芯片指示的后续测试,以及对健康人类淋巴细胞的选择性研究。
-
[EN] QUINAZOLINE DERIVATIVES, COMPOSITIONS, AND USES RELATED THERETO<br/>[FR] DÉRIVÉS DE QUINAZOLINE, COMPOSITIONS ET UTILISATIONS ASSOCIÉES申请人:UNIV EMORY公开号:WO2013181135A1公开(公告)日:2013-12-05The disclosure relates to quinazoline derivatives, compositions, and methods related thereto. In certain embodiments, the disclosure relates to inhibitors of NADPH-oxidases (Nox enzymes) and/or myeloperoxidase.
-
[EN] NOVEL THERAPEUTIC AGENTS FOR THE TREATMENT OF HBV INFECTION<br/>[FR] NOUVEAUX AGENTS THÉRAPEUTIQUES POUR LE TRAITEMENT DE L'INFECTION PAR HBV.申请人:NEWAVE PHARMACEUTICAL INC公开号:WO2018022282A1公开(公告)日:2018-02-01The disclosure includes compounds of Formula (I), wherein Z1, Z2, Z3, X, R1, R2, R3, R4, R5, R6, and R7 are defined herein. Also disclosed is a method for treating HBV infection.披露的内容包括式(I)的化合物,其中Z1、Z2、Z3、X、R1、R2、R3、R4、R5、R6和R7在此处定义。还披露了一种治疗HBV感染的方法。
-
[EN] ASH1L INHIBITORS AND METHODS OF TREATMENT THEREWITH<br/>[FR] INHIBITEURS DE ASH1L ET MÉTHODES DE TRAITEMENT AU MOYEN DE CEUX-CI申请人:UNIV MICHIGAN REGENTS公开号:WO2017197240A1公开(公告)日:2017-11-16Provided herein are small molecule inhibitors of ASH1L activity and small molecules that facilitate ASH1L degradation and methods of use thereof for the treatment of disease, including acute leukemia, solid cancers and other diseases dependent on activity of ASH1L.本文提供了ASH1L活性的小分子抑制剂,促进ASH1L降解的小分子以及它们的使用方法,用于治疗疾病,包括急性白血病、实体肿瘤和其他依赖于ASH1L活性的疾病。
-
Controlled-release compositions containing opioid agonist and antagonist申请人:——公开号:US20020010127A1公开(公告)日:2002-01-24Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of said opioid antagonist effective to attenuate a side effect of said opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.含有阿片激动剂、阿片拮抗剂和受控释放材料的控释剂型,其在给药间隔期间释放阿片激动剂的镇痛或亚镇痛量以及足以减轻所述阿片激动剂的副作用的阿片拮抗剂的量。当给予人类患者时,该剂型提供至少约8小时的镇痛作用。在其他实施例中,给药间隔期释放的拮抗剂剂量增强了阿片激动剂的镇痛效力。
表征谱图
-
氢谱1HNMR
-
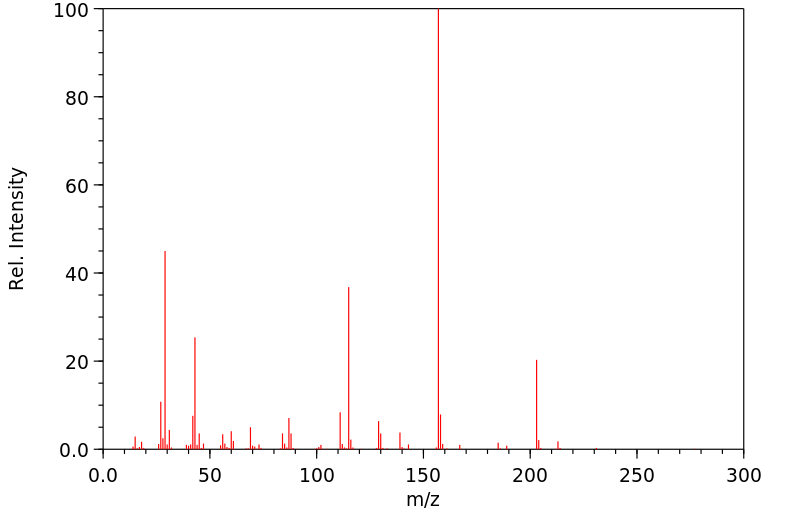
质谱MS
-
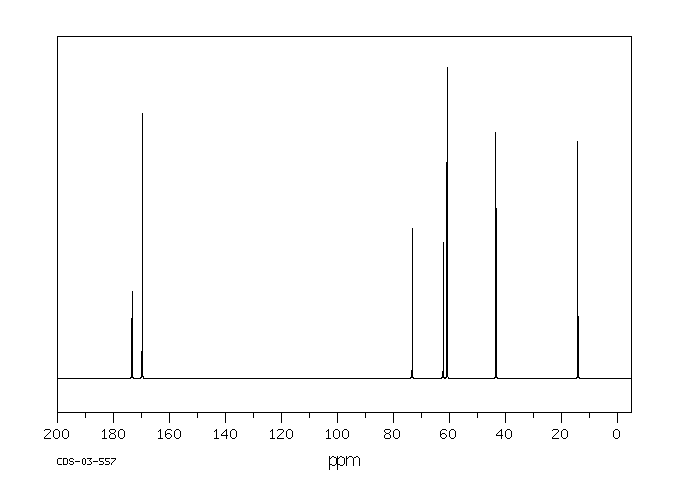
碳谱13CNMR
-
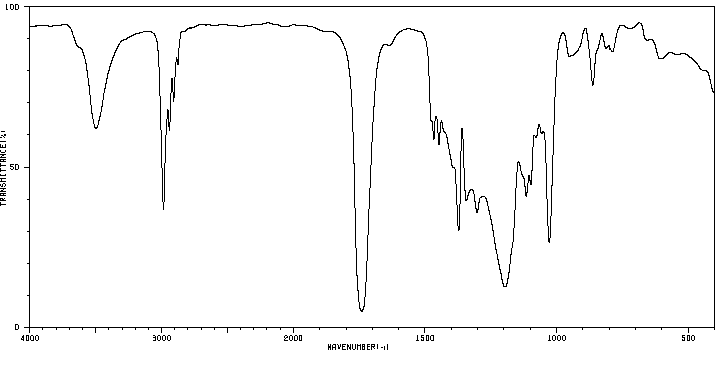
红外IR
-
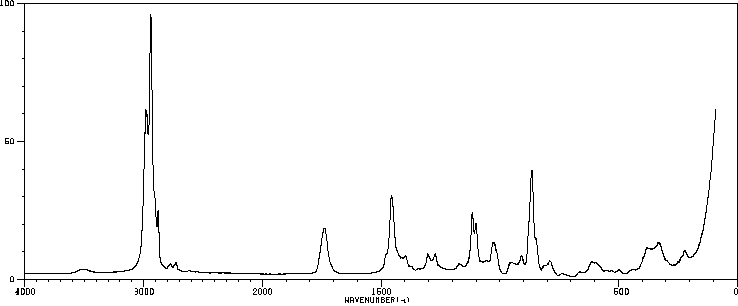
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸