三溴甲烷 | 75-25-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:8 °C
-
沸点:150 °C
-
密度:2.89 g/mL at 25 °C(lit.)
-
蒸气密度:8.7 (vs air)
-
闪点:148-150°C
-
溶解度:溶于水中的溶解度:800份(lit.)
-
暴露限值:NIOSH REL: TWA 0.5 ppm (5 mg/m3), IDLH 85e respiratory irritation (Patnaik, 1992).
-
介电常数:4.4(20℃)
-
LogP:2.400
-
物理描述:Colorless to yellow liquid with a chloroform-like odor. [Note: A solid below 47°F.]
-
颜色/状态:Colorless heavy liquid
-
气味:Similar to chloroform
-
味道:Sweetish taste
-
蒸汽密度:8.7 (NTP, 1992) (Relative to Air)
-
蒸汽压力:5.4 mm Hg at 25 °C /extrapolated/
-
亨利常数:5.35e-04 atm-m3/mole
-
稳定性/保质期:
-
分解:Hazardous decomposition products formed under fire conditions. - Carbon oxides, Hydrogen bromide gas.
-
粘度:0.74 mm²/s at 15 °C
-
腐蚀性:... Liquid bromoform will attack some forms of plastics, rubber, and coatings.
-
汽化热:39.66 kJ/mol at 149.1 °C; 46.05 kJ/mol at 25 °C
-
表面张力:41.53 dynes/cm at 20 °C
-
电离电位:10.48 eV
-
气味阈值:In water: detection: 0.3 mg/kg
-
折光率:Index of refraction: 1.5948 at 25 °C/D
-
保留指数:845.2;852.7;859;870;882;895;869.6;877;853.1;863.6;875;853;875;911;904.3;867.3;852;852;852;855;860
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 0.5 ppm (5 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:850 ppm
-
危险品标志:T
-
安全说明:S16,S28,S28A,S36/37,S45,S61,S7
-
危险类别码:R22,R51/53,R36/38,R23
-
WGK Germany:3
-
海关编码:2903399020
-
危险品运输编号:UN 2515 6.1/PG 3
-
危险类别:6.1
-
RTECS号:PB5600000
-
包装等级:III
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,库温不超过32℃,相对湿度不超过80%。 - 保持容器密封。 - 应与氧化剂、活性金属粉末、食用化学品分开存放,切忌混储。 - 储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 61562 |
| CAS: | 75-25-2 |
| 中文名称: | 三溴甲烷 |
| 英文名称: | tribromomethane;bromoform |
| 别 名: | 溴仿 |
| 分子式: | CHBr 3 |
| 分子量: | 252.77 |
| 熔 点: | 6~7℃ |
| 密 度: | 相对密度(水=1)2.89 |
| 蒸汽压: | |
| 溶解性: | 微溶于水,溶于乙醇、乙醚、氯仿、苯 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色重质液体,有似氯仿味 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作溶剂和有机合成中间体 |
2、对环境的影响:
遇碱分解,但在水体中则是高度持久性的化合物,不会被生物降解。特别中饮用水中会长期停留,从而造成危害。
一、健康危害
侵入途径:吸入、食入、经皮吸收。 健康危害:本品有麻醉和刺激作用,对肝脏有一定损害。轻度中毒有流泪、咽痒、头晕、头痛、无力。严重者有恶心、呕吐、昏迷、抽搐等。可致死。
二、毒理学资料及环境行为
毒性:三溴甲烷的毒性与二氯甲烷相类似,但毒性强度比二氯甲烷大。它能通过呼吸道、经口对人体严重毒害,也能经粘膜、眼睛甚至皮肤对人体严重毒害作用。亚急性和慢性毒性:大鼠吸入0.25mg/L,4小时/天,2个月,肝肾功能异常。 致突变性:微生物致突变:鼠伤寒沙门氏菌50ul/皿。
污染来源:三溴甲烷用作有机合成的中间体和药物制造。用溴甲烷作为消毒剂、镇痛剂、致冷剂、防火化学品的企业。以上工厂及企业在生产和使用三溴甲烷及贮运过程中的意外事故均会对环境造成危害。 代谢与降解:Lucas曾经在兔子试验中证实,通过直肠或吸入方式给予三溴甲烷后,部分在肝脏中被分解成代谢物,而后在兔子的组织内和排出的尿液内检出了无机溴化物。用三溴甲烷进行直肠麻醉后,可以从尿液中回收溴化钠-.3%-1.2%。环境中的三溴甲烷遇碱分解,但在环境水体中则是高度持久性的化合物,不会被生物降解。 危险特性:不燃。受高热分解产生有毒的溴化物气体。与锂、钾钠合金接触剧烈反应。 燃烧(分解)产物:一氧化碳、二氧化碳、溴化氢。
3、现场应急监测方法:快速检测管法;便携式气相色谱法《突发性环境污染事故应急监测与处理处置技术》万本太主编
4、实验室监测方法:| 监测方法 | 来源 | 类别 |
| 顶空气相色谱法 | GB/T17130-1997 | 水质 |
| 吹扫捕集-气相色谱法 | 中国环境监测总站 | 水质 |
| 气相色谱法 | 《固体废弃物试验与分析评价手册》中国环境监测总站等译 | 固体废弃物 |
| 色谱/质谱法 | 美国EPA525方法 | 水质 |
| 前苏联 | 车间空气中有害物质的最高容许浓度 | 5mg/m 3 |
| 前苏联 | 环境空气中有害物质的最高容许浓度 | 0.05mg/m 3 (日均值 ) |
| 中国(GHZB1-1999) | 地表水环境质量标准(I、II、III类水域) | 0.04mg/L |
6、应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。对于甲于三溴甲烷引起的土壤和水体的污染事故处置技术可参照三氯甲烷进行。⑴对于发生在地面上的污染事故及处置技术主要有:①迅速用土、沙子或其它可以取到的材料筑成坝以阻止液体的流动,特别要防止其流入附近的水体中,用土壤将其覆盖并将其吸收。也可以在其流动的下方向挖一坑,将其收集在坑内以防四处扩散,然后将液体收集到合适的容器中。②在处理过程中不要用铁器(如铁勺、铁容器、铁铲等),应改用其它工具,因为铁有助于三溴甲烷分解生成毒性更大的光气。有条件的话,操作人员在处理过程中应戴上防毒面具,或其它防护设备。③将受污染的土壤清除剥离后集中进行处理。操作应避免在光照条件下进行。⑵当三溴甲烷液体进入水体后,应设法阻断受污染水域与其它水域的通道,其方法为筑坝使其停止流动;开沟使其流向另一水体(如排污渠)等等。由于三溴甲烷属挥发性卤代烃类,对受其污染的水体最为简便易行处理方法是使用曝气(包括深进曝气)法,使其迅速从水体中逸散到大气中。另外,处理土壤的几种方法也可酌情使用。
二、防护措施
呼吸系统防护:空气中浓度超标时,应选择佩带自吸过滤式防毒面具(半面罩)。紧急事态抢救或撤离时,佩戴氧气呼吸器。 眼睛防护:一般不需要特殊防护,高浓度接触时可戴安全防护眼镜。 身体防护:穿透气型防毒服。 手防护:戴防化学品手套。 其它:工作现场禁止吸烟、进食和饮水。工作毕,沐浴更衣。单独存放被毒物污染的衣服。洗后备用。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。 眼睛接触:提起眼睑,用流动清水或生理盐水冲洗,就医。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 食入:饮足量温水,催吐,就医。
灭火方法:消防人员须佩戴防毒面具、穿全身消防服。灭火剂:泡沫、干粉、二氧化碳、砂土。
制备方法与用途
三溴甲烷是一种呼吸系统刺激物、麻醉剂以及肝脏毒物,可通过呼吸道、口腔或经由黏膜、眼睛甚至皮肤对人体造成严重伤害。短期过度暴露可导致眼、鼻、喉炎症;误吞可能引发头痛、头晕、口齿不清、意识模糊甚至死亡。长期接触则可能导致肝脏损伤、膀胱癌、结肠癌以及不良妊娠和发育。
化学性质三溴甲烷为无色重质液体,能与醇、苯、氯仿、醚、石油醚、丙酮及不挥发和易挥发的油类相互溶解,并可与多种有机溶剂形成共沸物。它在水中溶解度较低,熔点为7.7℃,沸点149.5℃,黏度(15℃)2.152mPa·s,相对密度2.8912(20/4℃),折光率1.6005(15℃)。这种物质不易燃易爆,具有轻微的氯仿气味和甜味。长期储存会逐渐分解成黄色液体,空气及光照可加速其分解过程,通常需添加4%乙醇作为稳定剂。
用途三溴甲烷可用作染料中间体、消毒剂、镇痛剂、麻醉剂、制冷剂、选矿剂、沉淀剂、溶剂和抗爆液组分等。此外,它还可用于测定分子量的溶剂,测定矿物的折射指数,并按比重分离各种矿石。在工业上可用于染料中间体、医药镇痛剂、空气熏蒸消毒剂、制冷剂、抗爆剂组分、树脂和石蜡的溶剂等。
生产方法三溴甲烷由丙酮与次溴酸钠反应制得。具体步骤为:先用溴素与碱液配制次溴酸钠,然后在碱性条件下将丙酮迅速加入-5℃至0℃的次溴酸钠中,保持温度在30-50℃左右,待温度下降后继续搅拌10分钟。随后,通过三溴甲酮分解生成溴仿,并进行静置分层、蒸馏、洗涤、过滤和干燥得到成品。
安全分类 类别有毒物质
毒性分级中毒
急性毒性大鼠口服LD50: 933毫克/公斤;小鼠口服LD50: 1072毫克/公斤
爆炸物危险特性与冠醚或KOH反应爆炸
可燃性危险特性不燃,高温分解时释放有毒溴化物烟雾
储运特性库房需通风低温干燥;应与食品添加剂分开存放
灭火剂 职业标准时间加权平均浓度(TWA)5毫克/立方米,短时暴露极限值(STEL)15毫克/立方米
上下游信息
反应信息
-
作为反应物:参考文献:名称:Electrocchemicai oxidatiob of ketones in methanol in the presence of alkali metal bromides摘要:Electrochemical oxidation of methyl ketones in methanol in the presence of alkali metal bromides affords methyl carboxylates. Benzyl alkyl ketones are transformed under similar conditions into methyl 3-phenylalkanoates, while ketones lacking alpha-benzyl or alpha-methyl group are oxidized into alpha-hydroxyketals.DOI:10.1016/s0040-4020(01)87078-2
-
作为产物:参考文献:名称:Cahours, Annales de Chimie (Cachan, France), 1847, vol. <3>19, p. 506摘要:DOI:
-
作为试剂:参考文献:名称:Cp2TiCl 催化的 2-O-THF 和 2-O-THP 醚对醇的意外温和保护揭示了溶剂在单电子转移反应中的有趣作用摘要:已经开发了一种在室温下将伯醇、仲醇和叔醇转化为相应的 THF 醚以及将伯醇和仲醇转化为相应的 THP 醚的方法,该方法使用由催化量的二氯化钛或( 4R,5R)-(–)-2,2-二甲基-α,α,α',α'-tetra(1-naphthyl)-1,3-dioxolane-4,5-dimethanolatotitanium(IV) 二氯化物:乙腈加合物与作为还原剂的锰(0)和作为溶剂的 THF 或 THP 中的溴仿一起使用。针对这种转变提出了一种自由基机制,揭示了溶剂在低价 TiIII 体系催化的单电子转移反应中的有趣作用。DOI:10.1002/ejoc.201500869
文献信息
-
Highly Regio- and Enantioselective Alkoxycarbonylative Amination of Terminal Allenes Catalyzed by a Spiroketal-Based Diphosphine/Pd(II) Complex作者:Jiawang Liu、Zhaobin Han、Xiaoming Wang、Zheng Wang、Kuiling DingDOI:10.1021/jacs.5b07764日期:2015.12.16An enantioselective alkoxycarbonylation-amination cascade process of terminal allenes with CO, methanol, and arylamines has been developed. It proceeds under mild conditions (room temperature, ambient pressure CO) via oxidative Pd(II) catalysis using an aromatic spiroketal-based diphosphine (SKP) as a chiral ligand and a Cu(II) salt as an oxidant and affords a wide range of α-methylene-β-arylamino
-
Palladium-Catalyzed Oxidative Carbonylative Coupling of Arylallenes, Arylboronic Acids, and Nitroarenes作者:Hui-Qing Geng、Jin-Bao Peng、Xiao-Feng WuDOI:10.1021/acs.orglett.9b02925日期:2019.10.18In this Letter, a palladium-catalyzed multicomponent procedure for the selective synthesis of α-substituted α,β-unsaturated ketones has been developed. With readily available allenes, arylboronic acids, and nitroarenes as the substrates, the reaction proceeds selectively to the desired α-substituted enones. Notably, no manipulation of carbon monoxide gas is needed here, and Mo(CO)6 has been applied
-
Diboration of 3-substituted propargylic alcohols using a bimetallic catalyst system: access to (<i>Z</i>)-allyl, vinyldiboronates作者:Cheryl L. Peck、Jan Nekvinda、Webster L. SantosDOI:10.1039/d0cc03563g日期:——
A Pd/Cu catalyst system facilitates the diboration of unactivated propargylic alcohols with pentafluoroboronic acid and diboron to generate (
Z )-allyl, vinyldiboronates. -
Macrolide Synthesis through Intramolecular Oxidative Cross-Coupling of Alkenes作者:Bing Jiang、Meng Zhao、Shu-Sen Li、Yun-He Xu、Teck-Peng LohDOI:10.1002/anie.201710601日期:2018.1.8A RhIII‐catalyzed intramolecular oxidative cross‐coupling between double bonds for the synthesis of macrolides is described. Under the optimized reaction conditions, macrocycles containing a diene moiety can be formed in reasonable yields and with excellent chemo‐ and stereoselectivity. This method provides an efficient approach to synthesize macrocyclic compounds containing a 1,3‐conjugated diene描述了用于大环内酯合成的双键之间的Rh III催化的分子内氧化交叉偶联。在优化的反应条件下,可以以合理的收率形成具有二烯部分的大环,并具有出色的化学和立体选择性。该方法为合成包含1,3-共轭二烯结构的大环化合物提供了一种有效的方法。
-
Copper-Catalyzed Difunctionalization of Allenes with Sulfonyl Iodides Leading to (<i>E</i>)-α-Iodomethyl Vinylsulfones作者:Ning Lu、Zhiguo Zhang、Nana Ma、Conghui Wu、Guisheng Zhang、Qingfeng Liu、Tongxin LiuDOI:10.1021/acs.orglett.8b01765日期:2018.7.20A highly regioselective iodosulfonylation of allenes in the presence of CuI and 1,10-phenanthroline has been developed for the synthesis of various useful (E)-α-iodomethyl vinylsulfones in moderate to excellent yields. This practical reaction is fast, operationally simple, and in particular, proceeds under very mild conditions to afford the target products with high regio- and stereoselectivity. The
表征谱图
-
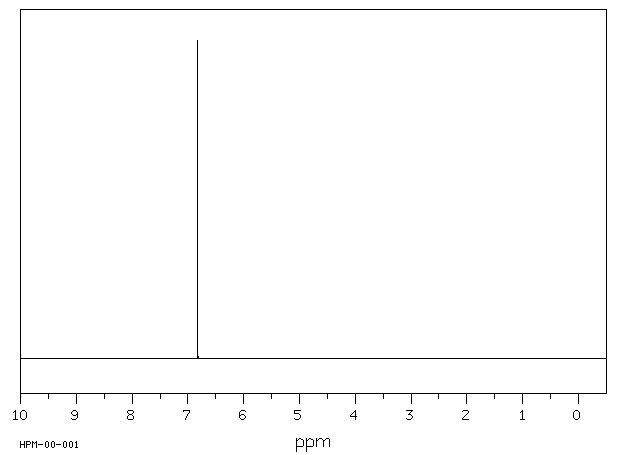
氢谱1HNMR
-
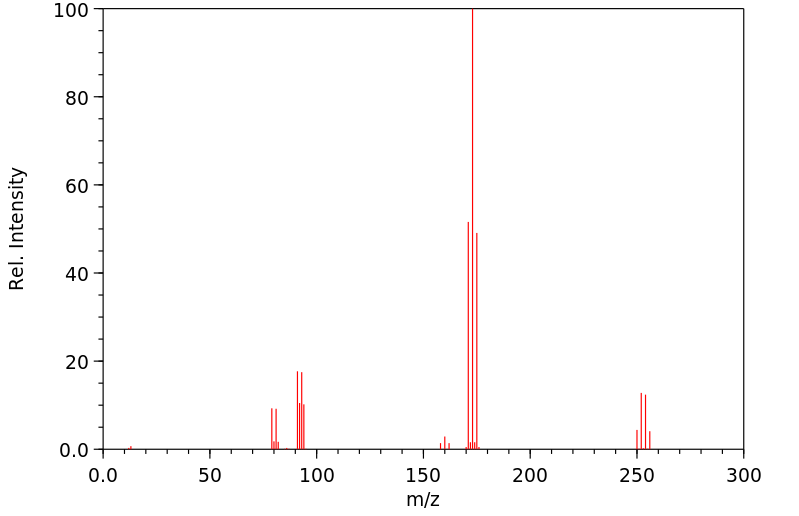
质谱MS
-
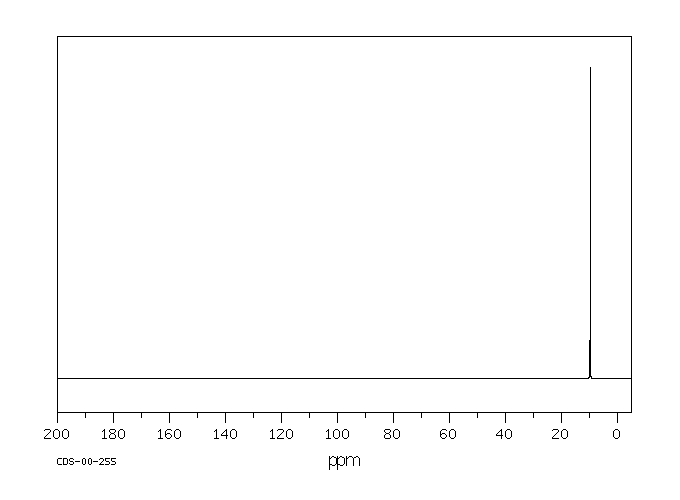
碳谱13CNMR
-
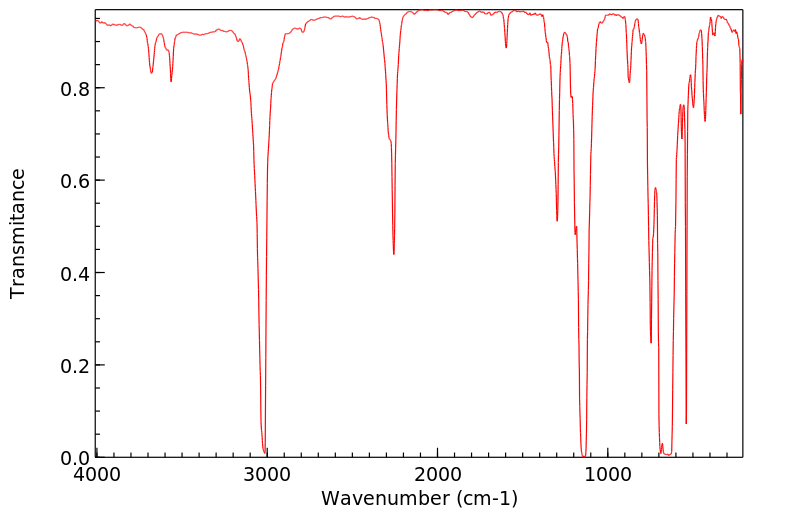
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息