1,1,4,4-甲苯基-1,3-丁二烯 | 1450-63-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:207-209 °C(lit.)
-
沸点:425.89°C (rough estimate)
-
密度:1.1108 (estimate)
-
溶解度:易溶于乙醇、苯、氯仿、甲苯和醋酸。
-
稳定性/保质期:
如果按照规格使用和储存,就不会分解,没有已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):8.6
-
重原子数:28
-
可旋转键数:5
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:2
-
海关编码:29029000
-
RTECS号:CY9040630
-
储存条件:密封,在2ºC至-8ºC下保存
SDS
| Name: | 1 1 4 4-Tetraphenyl-1 3-Butadiene Scintillation Grade 99+% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1450-63-1 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1450-63-1 | Benzene,1,1',1'',1'''-(1,3-butadiene-1 | 99+ | 215-914-7 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1450-63-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Needles
Color: white to yellow white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 196.00 - 198.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C28H22
Molecular Weight: 358.47
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1450-63-1: CY9040630 LD50/LC50:
CAS# 1450-63-1: Oral, mouse: LD50 = >14 gm/kg.
Carcinogenicity:
Benzene,1,1',1'',1'''-(1,3-butadiene-1,4-diylidene)tetrkis- - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1450-63-1: No information available.
Canada
CAS# 1450-63-1 is listed on Canada's NDSL List.
CAS# 1450-63-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1450-63-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-甲基-2,2-二苯基乙烯 1,1-diphenyl-1-propene 778-66-5 C15H14 194.276 3,3-二苯基丙烯腈 3,3-diphenylacrylonitrile 3531-24-6 C15H11N 205.259 β-苯基肉桂醛 3,3-diphenyl-2-propenal 1210-39-5 C15H12O 208.26 —— 3-bromo-1,1-diphenyl-1-propene 4801-15-4 C15H13Br 273.172 1,1-二苯乙烯 1,1-Diphenylethylen 530-48-3 C14H12 180.249 —— 3,3-diphenylacrylamide 4439-11-6 C15H13NO 223.274 3,3-二苯基-2-丙烯酸 3,3-diphenylacrylic acid 606-84-8 C15H12O2 224.259 (2-溴-1-苯基乙烯基)苯 1-bromo-2,2-diphenylethylene 13249-58-6 C14H11Br 259.145 —— 2-chloro-1,1-diphenylethene 4541-89-3 C14H11Cl 214.694 1,1'-(2,2-二溴-1,1-乙烯二基)二苯 1,1-dibromo-2,2-diphenylethylene 2592-73-6 C14H10Br2 338.041 —— 1,1,3,3-tetraphenyl-1-propene 4960-55-8 C27H22 346.472 四苯基丙二烯 tetraphenylallene 1674-18-6 C27H20 344.456 —— tetraphenylbutatriene 1483-68-7 C28H20 356.467 —— bis(2,2-diphenylvinyl) ether 93620-51-0 C28H22O 374.482 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,3-二苯基-2-丙烯酸 3,3-diphenylacrylic acid 606-84-8 C15H12O2 224.259
反应信息
-
作为反应物:描述:1,1,4,4-甲苯基-1,3-丁二烯 在 palladium hydroxide 10 wt. % on activated carbon 、 氢气 作用下, 以 甲醇 为溶剂, 20.0 ℃ 、303.99 kPa 条件下, 以99 %的产率得到1,1,4,4-四苯基丁烷参考文献:名称:空间相互作用中的激发态奇偶效应摘要:奇偶效应是自然界中一种奇妙的现象,它已被应用于有机自组装单分子层和液晶等多个领域。目前,每个奇偶效应的起源仍然难以捉摸,所有报道的奇偶效应都与基态特性有关。在这里,我们发现非共轭四苯基烷烃(TPA)的穿越空间相互作用(TSI)中存在激发态奇偶效应。具有偶数个烷基碳原子的TPA(C2-TPA、C4-TPA和C6-TPA)表现出强TSI、长波长发射和高QY。然而,奇数(C1-TPA、C3-TPA、C5-TPA 和 C7-TPA)几乎不存在,QY 可以忽略不计。系统的实验和理论结果表明,激发态奇偶效应是由烷基几何形状、分子运动性和分子间堆积三个因素综合决定的。此外,这些柔性发光TPA在荧光信息加密方面具有巨大的优势。这项工作将奇偶效应扩展到光物理学,证明了其在自然界中的重要性和普遍性。DOI:10.1021/jacs.3c08164
-
作为产物:参考文献:名称:One-pot synthesis of oligomeric aryl-substituted PPV analogs with extended π-conjugation摘要:我们研究了一种概念新颖的方法,即从相应的二元单体(n =  1)一步法合成具有 Hâ[CHC(Ar)âC6H4âC(Ar)CH]nâH (n =  2、4)型扩展Ï-共轭的低聚聚聚苯乙烯(PPV,â[C6H4âCHCH]â)类似物。通过重复三氟乙酸汞衍生物 Hâ[CHC(Ar)âC6H4âC(Ar)CH]nâHgCO2CF3 (n = 1, 2)的连续制备及其在 PdCl2 存在下的偶联,以高产率实现了低聚物化。这种方法的可行性体现在直接从相应的单体一锅制备出了几种四聚体。DOI:10.1039/a905710b
-
作为试剂:参考文献:名称:Oxyguanidines: application to non-peptidic phenyl-based thrombin inhibitors摘要:Although thrombin has been extensively researched with many examples of potent and selective inhibitors, the key characteristics of oral bioavailability and long half-life have been elusive. We report here a novel series non-peptidic phenyl-based, highly potent, highly selective and orally bioavailable thrombin inhibitors using oxyguanidines as guanidine-mimetics. (C) 2003 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0960-894x(03)00125-2
文献信息
-
The<i>in situ</i>-Generated Nickel(0)-catalyzed Homo-coupling of Alkenyl Halides with Zinc Powder. A Specific Outcome in Stereochemistry作者:Kentaro Takagi、Harutaka Mimura、Saburo InokawaDOI:10.1246/bcsj.57.3517日期:1984.12The catalytic activity of nickel(0) generated in situ from nickel(II) salt was examined in a dehalogenative coupling of alkenyl halides with zinc powder. The reaction of alkenyl bromides took place provided that potassium iodide was present to assist the reduction of nickel(II) with zinc powder, and also to convert the alkenyl bromides to the corresponding alkenyl iodides. A speculative view concerning
-
Ethics and Foreign Policy: The Antinomies of New Labour's ‘Third Way’ in Sub-Saharan Africa作者:Rita Abrahamsen、Paul WilliamsDOI:10.1111/1467-9248.00312日期:2001.6
This article explores how New Labour has attempted to implement its ideas about a ‘third way’ foreign policy in sub-Saharan Africa. Through an examination of British foreign policy practices, we explore whether New Labour has succeeded in finding a ‘third way’ between traditional views of socialism and capitalism in Africa. In particular, the article focuses on New Labour's attempts to build peace, prosperity and democracy on the African continent. We conclude that although New Labour's claims to add an ‘ethical dimension’ to foreign policy have succeeded in giving Britain a higher profile in the international arena, the implementation of such a policy is intrinsically difficult. These difficulties in turn arise from the antinomies embodied in New Labour's policy, or more specifically from the tension between the liberal internationalism of the third way and traditional concerns for the national interest, as well as the contradictions inherent in a commitment to both political and economic liberalism.
这篇文章探讨了新工党如何试图在撒哈拉以南非洲实施其关于“第三条道路”外交政策的想法。通过对英国外交政策实践的审查,我们探讨新工党是否成功地在非洲传统社会主义和资本主义观念之间找到了一种“第三条道路”。特别是,文章关注新工党在非洲大陆上建立和平、繁荣和民主的努力。我们得出结论,尽管新工党声称在外交政策中增加了“道德维度”成功地使英国在国际舞台上更加引人注目,但这种政策的实施本质上是困难的。这些困难反过来源于新工党政策所体现的矛盾,更具体地说,源于第三条道路的自由国际主义与对国家利益的传统关注之间的紧张关系,以及对政治和经济自由主义的承诺中固有的矛盾。 -
A metal-free heterogeneous photocatalyst for the selective oxidative cleavage of CC bonds in aryl olefins <i>via</i> harvesting direct solar energy作者:Yu Zhang、Nareh Hatami、Niklas Simon Lange、Emanuel Ronge、Waldemar Schilling、Christian Jooss、Shoubhik DasDOI:10.1039/d0gc01187h日期:——transition metal-free) to avoid further leaching in the final products. This is for sure a big challenge to an organic chemist and to the pharmaceutical industries! To make this feasible, a mild and efficient protocol has been developed using polymeric carbon nitrides (PCN) as metal-free heterogeneous photocatalysts to convert various olefins into the corresponding carbonyls. Later, this catalyst has beenC C键的选择性裂解对于合成含羰基的精细化学品和药物非常重要。新型方法,例如臭氧分解反应,Lemieux-Johnson氧化反应等。已经存在。与此平行,还发现了使用均相催化剂的催化方法。考虑到非均相催化剂的各种优点,例如可循环性和稳定性,已将几种基于过渡金属的非均相催化剂用于该反应。但是,制药行业更喜欢使用不含金属的催化剂(尤其是不含过渡金属的催化剂),以避免最终产品中进一步浸出。对于有机化学家和制药行业来说,这无疑是一个巨大的挑战!为了使之可行,已经开发了一种温和而有效的方案,使用聚合碳氮化物(PCN)作为无金属的非均相光催化剂,将各种烯烃转化为相应的羰基。后来,该催化剂已被用于使用直接太阳能的克级合成药物中。详细的机械研究揭示了氧气,催化剂和光源的实际作用。
-
Ni(0)-Triphenylphosphine Complex-Catalyzed Homo-Coupling of 1-Alkenyl Halides with Zinc Powder作者:Ken Sasaki、Kikuji Nakao、Yoshihiko Kobayashi、Mutsuji Sakai、Norito Uchino、Yasumasa Sakakibara、Kentaro TakagiDOI:10.1246/bcsj.66.2446日期:1993.8The homo-coupling of 1-alkenyl halides was examined in the presence of NiBr2(PPh3)2, PPh3, and excess zinc. The reactions proceed under very mild conditions to give high yields of conjugated dienes. The addition of KI or thiourea was unnecessary for a successful reaction, in contrast with systems without an external phosphine ligand.
-
Visible‐Light‐Triggered, Metal‐ and Photocatalyst‐Free Acylation of <i>N</i> ‐Heterocycles作者:Lucas Guillemard、Françoise Colobert、Joanna Wencel‐DelordDOI:10.1002/adsc.201800692日期:2018.11.5photoinduced acylation of N‐heterocycles is explored. This visible‐light triggered reaction occurs not only under extremely mild reaction conditions, but also does not require the presence of a photosensitizer. The mechanistic studies suggest formation of EDA complexes prompt to harness the energy from visible‐light. Compatibility with a large panel of α‐keto acids as acyl precursors and an array of N‐heterocycles
表征谱图
-
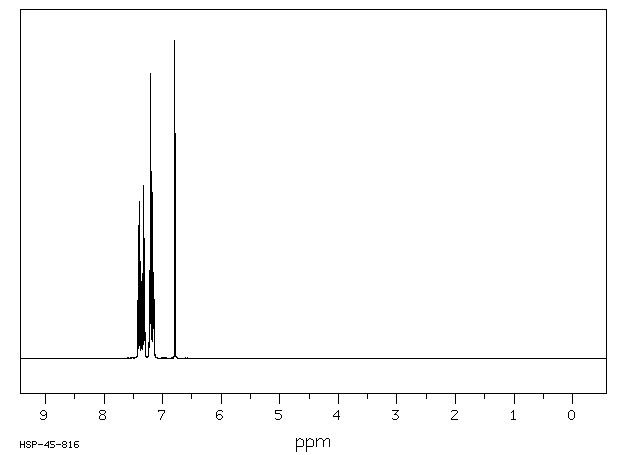
氢谱1HNMR
-
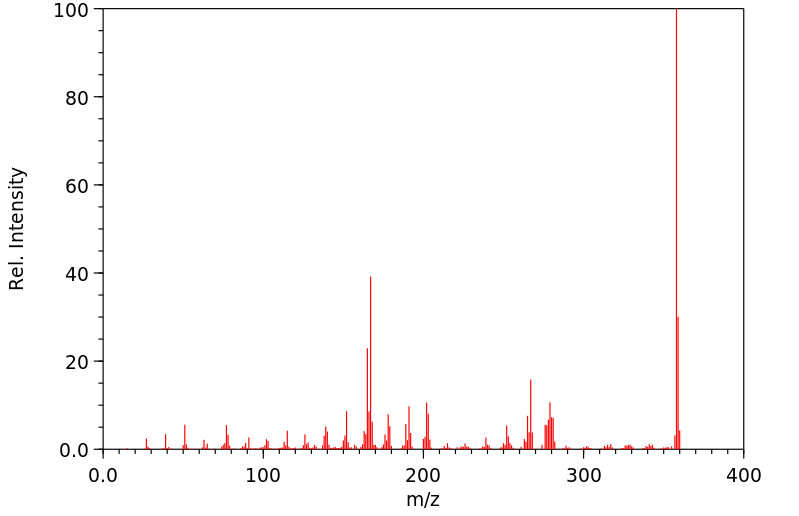
质谱MS
-
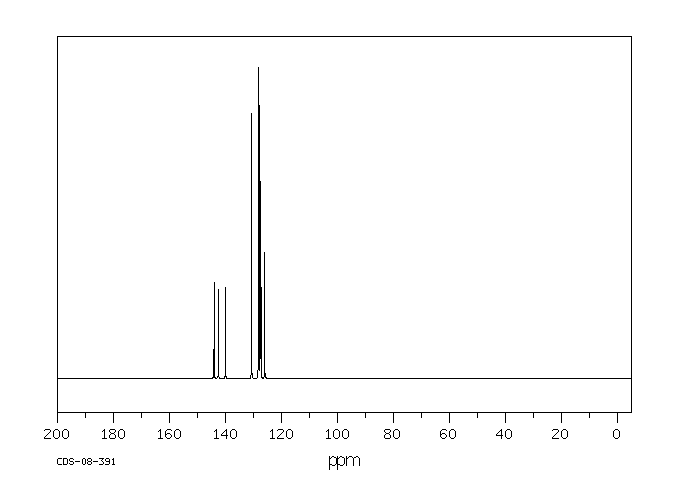
碳谱13CNMR
-
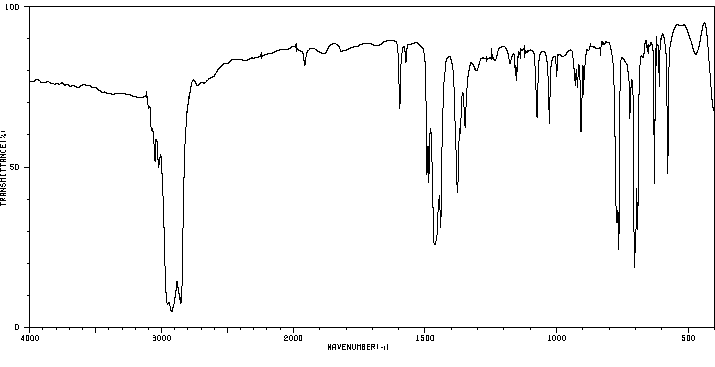
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息