己烯醛 | 505-57-7
中文名称
己烯醛
中文别名
2-己烯醛;2-己醛
英文名称
2-hexenal
英文别名
hex-2-enal;hexenal;2-hexen-1-al;hexen-2-al
CAS
505-57-7;1335-39-3
化学式
C6H10O
mdl
MFCD00007008
分子量
98.1448
InChiKey
MBDOYVRWFFCFHM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-66°C (estimate)
-
沸点:133.68°C (estimate)
-
密度:0.8445
-
LogP:1.58
-
物理描述:Pale yellow to colourless oily liquid; Strong fruity, green, vegetable-like aroma
-
溶解度:Soluble in propylene glycol and most fixed oils; Very slightly soluble in water
-
折光率:1.443-1.449
-
保留指数:860;838;843;839;828.7;826;839;832;835;854;857;840;832;827;820;866;835;826;824;824;816;841;817
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2912190090
SDS
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:α,β-不饱和醛的催化对映选择性过氧化,用于不对称合成生物学上重要的手性内过氧化物摘要:我们开发了一种前所未有的高度对映选择性催化过氧化烯醛。这一发展的关键是发现改变氢过氧化物的结构对有机催化不对称过氧化的对映选择性有显着影响。这种新的转化使得开发了一种对映选择性路线,通向抗癌天然产物 stolonoxide 家族所有成员共享的核心结构,即连接的反式-3,6-二取代-1,2-二恶烷和反式-2,5-二取代-四氢呋喃环系。我们的路线还具有前所未有的手性双(环氧)氢过氧化物环化级联。在这项工作中建立的新方法和合成策略应适用于广泛范围的手性 1,2-二氧戊环和 1,2-二氧六环的对映选择性合成,DOI:10.1021/jacs.5b05345
-
作为产物:描述:参考文献:名称:对甲氧基-1-己烯-1-yne-3和甲氧基-1-己二烯-1,3进行酸水解。取代的吡唑和顺己烯-3-醛的合成摘要:1-甲氧基-1-己烯-3-炔(I)的酸水解产生的主要产物是不稳定的β-氧己醛,而不是Herbertz先前提出的β-己醛。简要讨论了该反应的机理。在苯肼存在下酸水解I,生成两种1-苯基-预防性吡唑的混合物。用肼得到3-丙基-吡唑。该反应对于3-取代的吡唑的合成是普遍感兴趣的。DOI:10.1002/hlca.19630460531
文献信息
-
Vinylogous hydrazone strategy for the organocatalytic alkylation of heteroaromatic derivatives作者:Beata Łukasik、Justyna Kowalska、Sebastian Frankowski、Łukasz AlbrechtDOI:10.1039/d1cc01923f日期:——umpolung approach for the asymmetric Friedel–Crafts-type alkylation of electron-poor heteroaromatic systems has been developed. It is based on the vinylogous reactivity of hydrazones derived from heteroaromatic aldehydes. The donating effect of the hydrazone moiety can be efficiently transferred over the heteroaromatic system activating it towards an asymmetric Friedel–Crafts reaction with α,β-unsaturated
-
Thermoregulated phase-transfer iridium nanoparticle catalyst: highly selective hydrogenation of the CO bond for α,β-unsaturated aldehydes and the CC bond for α,β-unsaturated ketones作者:Wenjiang Li、Yanhua Wang、Pu Chen、Min Zeng、Jingyang Jiang、Zilin JinDOI:10.1039/c6cy01137c日期:——thermoregulated ligand Ph2P(CH2CH2O)22CH3-stabilized iridium nanoparticles exhibited a totally different orientation for the hydrogenation of unsaturated carbonyl compounds, namely, highly selective hydrogenation of the CO bond for α,β-unsaturated aldehydes and the CC bond for α,β-unsaturated ketones.
-
Nickel-catalyzed Conjugate Addition of Arylboron Reagents to α,β-Unsaturated Carbonyl Compounds with the Aid of a Catalytic Amount of an Alkyne作者:Eiji Shirakawa、Yuichi Yasuhara、Tamio HayashiDOI:10.1246/cl.2006.768日期:2006.7Alkynes in combination with a catalytic amount of a nickel complex were found to catalyze the conjugate addition of arylboron reagents to α,β-unsaturated carbonyl compounds, where use of an optical...
-
A biocatalytic method for the chemoselective aerobic oxidation of aldehydes to carboxylic acids作者:Tanja Knaus、Vasilis Tseliou、Luke D. Humphreys、Nigel S. Scrutton、Francesco G. MuttiDOI:10.1039/c8gc01381k日期:——optimised biocatalytic oxidation runs in phosphate buffer at pH 8.5 and at 40 °C. From a set of sixty-one aliphatic, aryl-aliphatic, benzylic, hetero-aromatic and bicyclic aldehydes, fifty were converted with elevated yield (up to >99%). The exceptions were a few ortho-substituted benzaldehydes, bicyclic heteroaromatic aldehydes and 2-phenylpropanal. In all cases, the expected carboxylic acid was shown在此,我们提出了一项使用三种重组醛脱氢酶(ALDH)将醛氧化为羧酸的研究。ALDH 以纯化形式与烟酰胺氧化酶 (NOx) 一起使用,该酶以双氧(大气压下的空气)为代价回收催化 NAD + 。为了更方便的实际应用,还使用冻干全细胞和静息细胞生物催化剂研究了该反应。优化的生物催化氧化在 pH 8.5 和 40 °C 的磷酸盐缓冲液中运行。从一组 61 种脂肪族、芳基脂肪族、苄基、杂芳族和双环醛中,其中 50 种以较高的产率转化(高达 >99%)。少数邻位取代的苯甲醛、双环杂芳醛和2-苯基丙醛除外。在所有情况下,预期的羧酸都是唯一的产物(>99% 化学选择性)。同一分子内的其他可氧化官能团(例如羟基、烯烃和杂芳族氮或硫原子)保持不变。该反应规模用于氧化 5-(羟甲基)糠醛 (2 g)(一种生物基原料),得到 5-(羟甲基)糠酸,分离收率为 61%。新的生物催化方法避免使用有毒或不安全的氧化剂、强
-
Regio- and <i>Trans</i>-Selective Ni-Catalyzed Coupling of Butadiene, Carbonyls, and Arylboronic Acids to Homoallylic Alcohols under Base-Free Conditions作者:Yu-Qing Li、Guang Chen、Shi-Liang ShiDOI:10.1021/acs.orglett.1c00488日期:2021.4.2We herein report a Ni-catalyzed three-component coupling of 1,3-butadiene, carbonyl compounds, and arylboronic acids as a general synthetic approach to 1,4-disubstituted homoallylic alcohols, an important class of compounds, which have previously not been straightforward to access. The reaction occurs efficiently using a Ni(cod)2 catalyst without any external base and ligand at ambient temperature
表征谱图
-
氢谱1HNMR
-
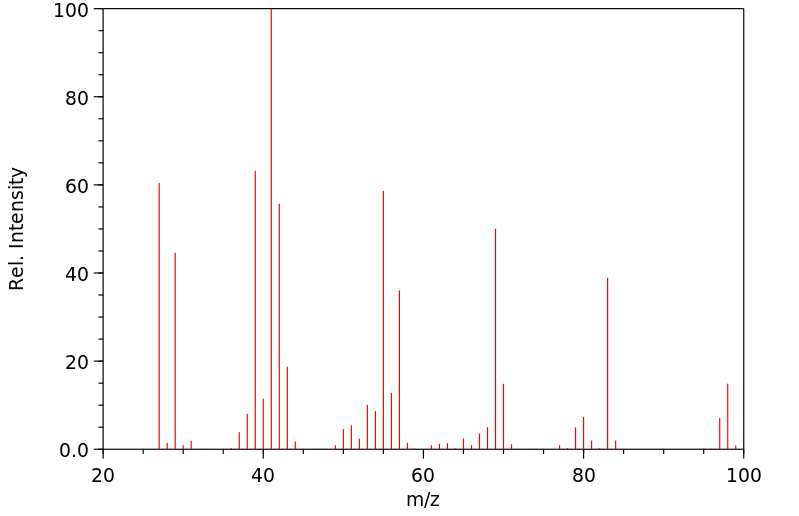
质谱MS
-
碳谱13CNMR
-
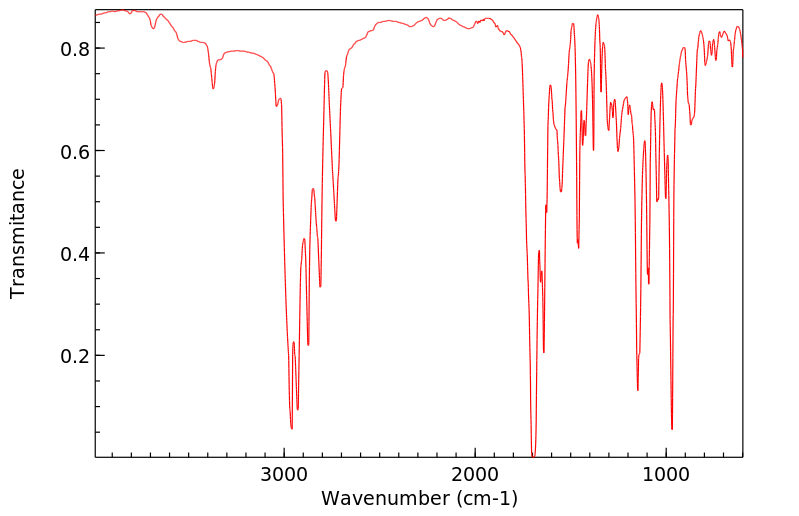
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷