5-氯-1-甲基咪唑 | 872-49-1
中文名称
5-氯-1-甲基咪唑
中文别名
1-甲基-5-氯咪唑
英文名称
N-methyl-5-chloroimidazole
英文别名
5-chloro-1-methylimidazole
CAS
872-49-1
化学式
C4H5ClN2
mdl
MFCD00014505
分子量
116.55
InChiKey
NYDGOZPYEABERA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:82-85°C(11 mmHg)
-
沸点:82-85 °C/11 mmHg (lit.)
-
密度:1.25 g/mL at 25 °C (lit.)
-
闪点:205 °F
-
溶解度:溶于氯仿,不溶于水
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:17.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
安全说明:S26,S37/39
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:2933290090
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将容器密封保存,并储存在阴凉、干燥的地方。
SDS
5-氯-1-甲基咪唑 修改号码:6
模块 1. 化学品
产品名称: 5-Chloro-1-methylimidazole
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5-氯-1-甲基咪唑
百分比: >98.0%(GC)(T)
CAS编码: 872-49-1
分子式: C4H5ClN2
5-氯-1-甲基咪唑 修改号码:6
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
热敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
5-氯-1-甲基咪唑 修改号码:6
模块 9. 理化特性
外观: 透明
颜色: 无色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 85 °C/1.5kPa
闪点: 96°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.26
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
5-氯-1-甲基咪唑 修改号码:6
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 5-Chloro-1-methylimidazole
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5-氯-1-甲基咪唑
百分比: >98.0%(GC)(T)
CAS编码: 872-49-1
分子式: C4H5ClN2
5-氯-1-甲基咪唑 修改号码:6
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
热敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
5-氯-1-甲基咪唑 修改号码:6
模块 9. 理化特性
外观: 透明
颜色: 无色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 85 °C/1.5kPa
闪点: 96°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.26
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
5-氯-1-甲基咪唑 修改号码:6
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯咪唑 4-chloro-1H-imidazole 15965-31-8 C3H3ClN2 102.523
反应信息
-
作为反应物:描述:参考文献:名称:Wallach; Schulze, Chemische Berichte, 1881, vol. 14, p. 422摘要:DOI:
-
作为产物:描述:参考文献:名称:Wallach, Boehringer, Justus Liebigs Annalen der Chemie, 1877, vol. 184, p. 51摘要:DOI:
-
作为试剂:描述:Beta-pinene 在 5-氯-1-甲基咪唑 、 iron(III) chloride hexahydrate 、 双氧水 作用下, 以 2-甲基-2-丁醇 、 水 为溶剂, 反应 1.0h, 生成 4-(2-羟基-2-丙基)环己烯-1-甲醇 、 紫苏醇 、 桃金娘烯醇参考文献:名称:Design of a bio-inspired imidazole-based iron catalyst for epoxidation of olefins: Mechanistic insights摘要:A novel defined iron catalyst for the epoxidation of aromatic and aliphatic olefins with hydrogen peroxide as the terminal oxidant is described Our catalyst approach is based on bio-inspired both alkyl- and aryl-substituted imidazoles in combination with cheap and abundant iron trichloride hexahydrate Heterocycles similar to imidazole can be used as ligands in this epoxidation system too The novel system is stable towards air and water It is shown that the mechanism depends strongly on the used ligands and substrates In the presence of radical scavengers no carbon-centered radical could be detected (C) 2010 Elsevier B V All rights reservedDOI:10.1016/j.cattod.2010.04.034
文献信息
-
Discovery of 3-Chloro-<i>N</i>-{(<i>S</i>)-[3-(1-ethyl-1<i>H</i>-pyrazol-4-yl)phenyl][(2<i>S</i>)-piperidine-2-yl]methyl}-4-(trifluoromethyl)pyridine-2-carboxamide as a Potent Glycine Transporter 1 Inhibitor作者:Shuji Yamamoto、Tsuyoshi Shibata、Kumi Abe、Koji Oda、Takeshi Aoki、Yasunori Kawakita、Hiroshi KawamotoDOI:10.1248/cpb.c16-00314日期:——inhibitory activity. Starting from 2-chloro-N-(S)-phenyl[(2S)-piperidin-2-yl]methyl}-3-(trifluoromethyl)benzamide (2, SSR504734), the introduction of heteroaromatic rings enabled an increase in the GlyT1 inhibitory activity. Subsequent optimization led to the identification of 3-chloro-N-(S)-[3-(1-ethyl-1H-pyrazol-4-yl)phenyl][(2S)-piperidine-2-yl]methyl}- 4-(trifluoromethyl)pyridine-2-carboxamide (7w)
-
Imidazole compounds and their use as transglutaminase inhibitors
-
A substituent- and temperature-controllable NHC-derived zwitterionic catalyst enables CO<sub>2</sub> upgrading for high-efficiency construction of formamides and benzimidazoles作者:Zhaozhuo Yu、Zhengyi Li、Lilong Zhang、Kaixun Zhu、Hongguo Wu、Hu Li、Song YangDOI:10.1039/d1gc01897c日期:——zwitterionic catalyst for efficient CO2 reductive upgrading via either N-formylation or further coupling with cyclization under mild conditions (25 °C, 1 atm CO2) using hydrosilane as a hydrogen source. More than 30 different alkyl and aromatic amines could be transformed into the corresponding formamides or benzimidazoles with remarkable yields (74%–98%). The electronic effect of the introduced substituent近年来,将温室气体CO 2化学催化升级为有价值的化学品和生物燃料引起了广泛关注。在已报道的方法中,CO 2与胺的N-甲酰化由于其在构建含N 线性和环状骨架方面的多功能性而具有重要意义。这里,稳定的N-杂环卡宾-羧基加合物(NHC-CO 2)中制备的轻便和可作为用于高效CO可回收两性离子催化剂2还原升级经由任一Ñ -formylation或另外的耦合用温和的条件下环化(25 °C, 1 atm CO 2) 使用氢硅烷作为氢源。超过 30 种不同的烷基和芳香胺可以转化为相应的甲酰胺或苯并咪唑,产率显着(74%–98%)。发现引入的取代基对NHC-CO 2的电子效应明显影响两性离子催化剂的热稳定性和亲核性,这与其催化活性直接相关。此外,NHC-CO 2可以通过在特定温度下原位脱羧来提供CO 2,这取决于引入的取代基类型。实验和计算研究表明,NHC-CO 2上的羧基物质不仅是亲核中心,而且还是在氢化硅烷化过程中快速捕获或替代环境
-
Modulating Reactivity and Diverting Selectivity in Palladium-Catalyzed Heteroaromatic Direct Arylation Through the Use of a Chloride Activating/Blocking Group作者:Benoît Liégault、Ivan Petrov、Serge I. Gorelsky、Keith FagnouDOI:10.1021/jo902515z日期:2010.2.19Through the introduction of an aryl chloride substituent, the selectivity of palladium-catalyzed direct arylation may be diverted to provide alternative regioisomeric products in high yields. In cases where low reactivity is typically observed, the presence of the carbon−chlorine bond can serve to enhance reactivity and provide superior outcomes. From a strategic perspective, the C−Cl bond is easily
-
Nucleophilic C–H Etherification of Heteroarenes Enabled by Base-Catalyzed Halogen Transfer作者:Thomas R. Puleo、Danielle R. Klaus、Jeffrey S. BandarDOI:10.1021/jacs.1c06481日期:2021.8.18for the direct C–H etherification of N-heteroarenes. Potassium tert-butoxide catalyzes halogen transfer from 2-halothiophenes to N-heteroarenes to form N-heteroaryl halide intermediates that undergo tandem base-promoted alcohol substitution. Thus, the simple inclusion of inexpensive 2-halothiophenes enables regioselective oxidative coupling of alcohols with 1,3-azoles, pyridines, diazines, and polyazines
表征谱图
-
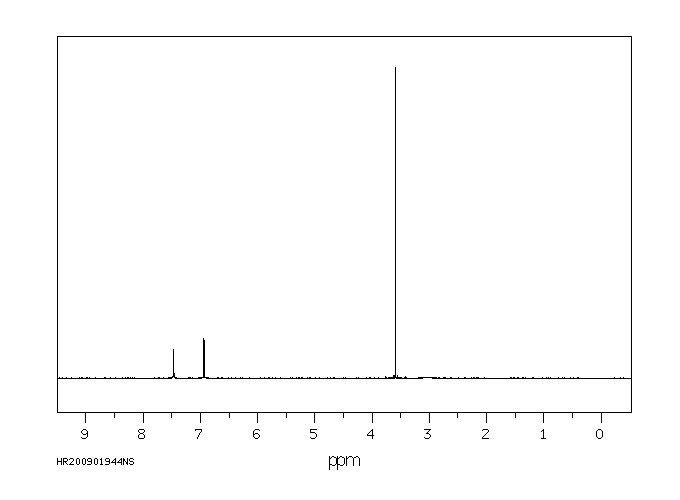
氢谱1HNMR
-
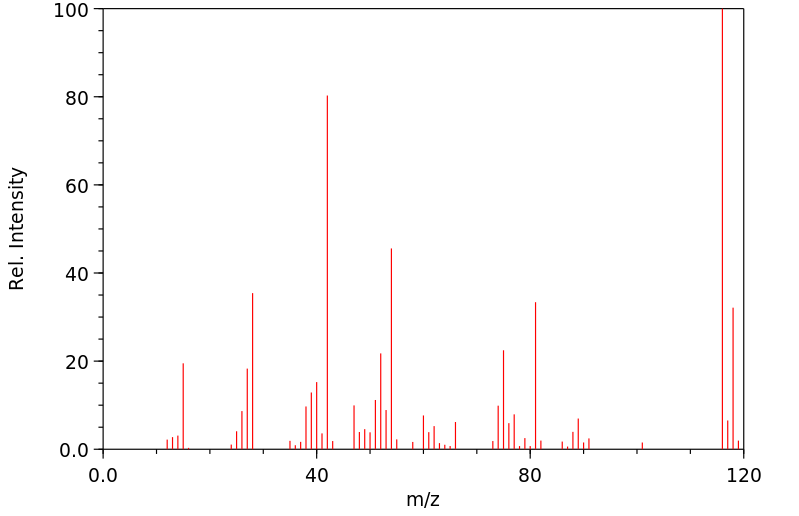
质谱MS
-
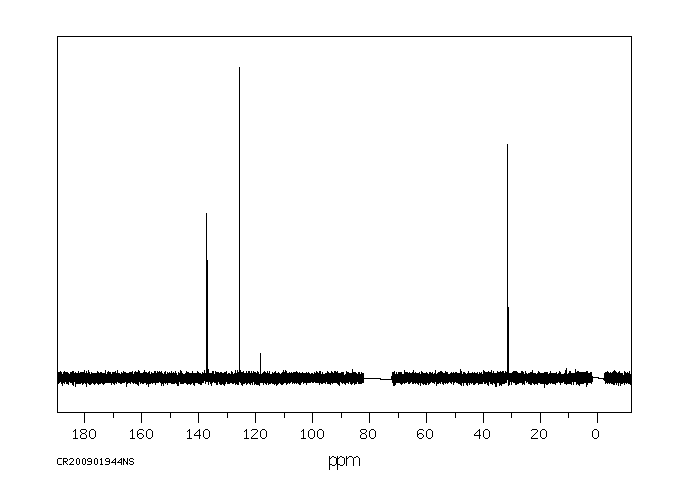
碳谱13CNMR
-
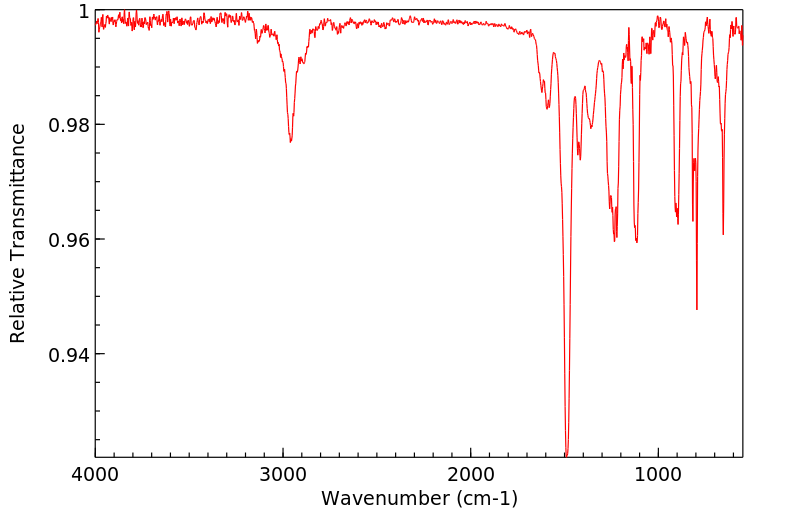
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
试剂2,5-Dibromo-3,4-dihexylthiophene
苯-1,2,4-三羧酸-丙烷-1,2,3-三醇(1:1)
碘吡咯
癸氯-二茂铁
甲酮,(4,5-二溴-1H-吡咯-2-基)苯基-
甲基3-氟-1H-1,2,4-三唑-5-羧酸酯
溴代二茂铁
溴-(3-溴-2-噻嗯基)镁
派瑞林D
派瑞林 F 二聚体
氯代二茂铁
曲洛酯
异噻唑,3-氯-5-甲基-
地茂酮
四碘硒吩
四碘噻吩
四碘呋喃
四溴噻吩
四溴吡咯
四溴-N-甲基吡咯
四氯噻吩
四氟噻吩
噻菌腈
噻美尼定.
噻吩,3-溴-4-(1-辛炔基)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(Z)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(E)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(E)-
噻吩,2,5-二氯-3,4-二(氯甲基)-
喷贝特
咪唑烷,2-(4-溴-5-甲基-2-呋喃基)-1,3-二甲基-
叔丁基2-溴-4,6-二氢-5H-吡咯并[3,4-D]噻唑-5-羧酸酯
叔-丁基3-溴-6,7-二氢-1H-吡唑并[4,3-C]吡啶-5(4H)-甲酸基酯
叔-丁基2-溴-5,6-二氢咪唑并[1,2-A]吡嗪-7(8H)-甲酸基酯
叔-丁基(4-溴-5-氰基-1-甲基-1H-吡唑-3-基)氨基甲酯
双环[4.2.0]辛-1,3,5-三烯-7-甲腈,2-氟-
八氟联苯烯
八氟二苯并硒吩
全氟苯并环丁烯二酮
二苯基氯化碘盐
二联苯碘硫酸盐
二氯对二甲苯二聚体
二氯[2-甲基-3(2H)-异噻唑酮-O]的钙合物
二氯-1,2-二硫环戊烯酮
二-(3-溴-1,2,4-噻二唑-5-基)-二硫醚
二(2-噻吩基)碘鎓
乙酸,[[[1-(3-溴-5-异[口噁]唑基)亚乙基]氨基]氧代]-,甲基酯,(E)-
[四丁基铵][Δ-三(四氯-1,2-苯二醇酸根)磷酸盐(V)]
[3-(4-氯-3,5-二甲基-1H-吡唑-1-基)丙基]胺
[3-(4-氯-1H-吡唑-1-基)-2-甲基丙基]胺