5,5-二甲己烷2,4-二酮 | 7307-04-2
中文名称
5,5-二甲己烷2,4-二酮
中文别名
5,5-二甲己烷-2,4-二酮;5,5-二甲基己烷-2,4-二酮
英文名称
acetylpinacolin
英文别名
5,5-dimethylhexane-2,4-dione
CAS
7307-04-2
化学式
C8H14O2
mdl
——
分子量
142.198
InChiKey
LCLCVVVHIPPHCG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:105-106 °C
-
沸点:165 °C
-
密度:0.912 g/mL at 25 °C
-
闪点:57°C
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:3
-
危险品标志:Xn
-
安全说明:S23,S24/25
-
危险类别码:R22,R10,R
-
WGK Germany:3
-
海关编码:29141990
-
危险品运输编号:UN 1224
-
包装等级:III
-
储存条件:请将密封储存于阴凉干燥环境中。
SDS
Section 1: Product Identification
Chemical Name: 2,2-Dimethyl-3,5-hexanedione, min. 97%
CAS Registry Number: 7307-04-2
Formula: (CH3)3CC(O)CH2C(O)CH3
EINECS Number: 230-760-0
Chemical Family: organic compound
Synonym: 5,5-dimethylhexane-2,4-dione
Section 2: Composition and Information on Ingredients
Ingredient CAS Number Percent ACGIH (TWA) OSHA (PEL)
Title Compound 7307-04-2 100% no data no data
Section 3: Hazards Identification
Emergency Overview: Irritating to skin, eyes and mucous membranes. May be harmful if swallowed.
Primary Routes of Exposure: Ingestion ,inhalation, skin, eyes
Eye Contact: Irritating to eyes. May cause redness and pain.
Skin Contact: Irritating to skin. May cause itching and redness.
Inhalation: Irritating to the nose, mucous membranes and respiratory tract.
Ingestion: No specific information is available on the physiological effects of ingestion.
Acute Health Affects: Irritating to skin, eyes, and mucous membranes.
Chronic Health Affects: No information available on long-term chronic effects.
NTP: No
IARC: No
OSHA: No
SECTION 4: First Aid Measures
Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need
Eye Exposure:
assistance in keeping their eye lids open. Get immediate medical attention.
Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
Skin Exposure:
irritation persists.
Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty
Inhalation:
in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance.
Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
Ingestion:
vomiting only if directed by medical personnel.
SECTION 5: Fire Fighting Measures
Flash Point: not applicable
Autoignition Temperature: no data
Explosion Limits: no data
Extinguishing Medium: dry chemical, carbon dioxide, or foam.
If this product is involved in a fire, fire fighters should be equipped with a NIOSH approved positive pressure
Special Fire Fighting Procedures:
self-contained breathing apparatus and full protective clothing.
Hazardous Combustion and If involved in a fire this material may release toxic and corrosive fumes.
Decomposion Products:
Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards.
SECTION 6: Accidental Release Measures
Small spills can be mixed with vermiculite, sodium carbonate or other suitable non combustible adsorbent and
Spill and Leak Procedures:
swept up.
SECTION 7: Handling and Storage
Handling and Storage: Store the material in a tightly sealed container in a cool, dry place.
SECTION 8: Exposure Controls and Personal Protection
Eye Protection: Always wear approved safety glasses when handling a chemical substance in the laboratory.
Skin Protection: Wear appropriate chemical resistant gloves and clothing.
Ventilation: If possible, handle the material in an efficient fume hood.
If ventilation is not available a respirator should be worn. The use of respirators requires a Respirator
Respirator:
Protection Program to be in compliance with 29 CFR 1910.134.
Ventilation: If possible, handle the material in an efficient fume hood.
Additional Protection: No additional protection required.
SECTION 9: Physical and Chemical Properties
Color and Form: colorless liquid
Molecular Weight: 142.2
Melting Point: no data
Boiling Point: no data
Vapor Pressure: no data
Specific Gravity: no data
Odor: not determined
Solubility in Water: insoluble
SECTION 10: Stability and Reactivity
Stability: air and moisture stable
Hazardous Polymerization: no hazardous polymerization
Conditions to Avoid: none
Incompatibility: oxidizing agents
Decomposition Products: carbon monoxide, carbon dioxide and organic fumes.
SECTION 11: Toxicological Information
RTECS Data: No information available in the RTECS files.
Carcinogenic Effects: no data
Mutagenic Effects: no data
Tetratogenic Effects: no data
SECTION 12: Ecological Information
Ecological Information: No information available
SECTION 13: Disposal Considerations
Disposal: Dispose of according to federal, state, and local regulations.
SECTION 14: Transportation
Shipping Name (CFR): Non-hazardous
Hazard Class (CFR): NA
Additional Hazard Class (CFR): NA
Packaging Group (CFR): NA
UN ID Number (CFR): NA
Shipping Name (IATA): Non-hazardous
Hazard Class (IATA): NA
Additional Hazard Class (IATA): NA
Packaging Group (IATA): NA
UN ID Number (IATA): NA
SECTION 15: Regulatory Information
TSCA: Not listed in the TSCA inventory.
SARA (Title 313): Title compound not listed.
Second Ingredient: none
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,2-二甲基己酮 2,2,-dimethylhexan-3-one 5405-79-8 C8H16O 128.214 —— α-methylacetylpinacoline 60600-51-3 C9H16O2 156.225 —— 5,5-dimethyl-hexane-2,3,4-trione 54458-36-5 C8H12O3 156.181 —— 3-Chlor-5,5-dimethyl-2,4-hexandion 41564-69-6 C8H13ClO2 176.643 —— 5-hydroxy-2,2-dimethyl-hexan-3-one 173343-33-4 C8H16O2 144.214
反应信息
-
作为反应物:描述:参考文献:名称:Weygand; Baumgaertel, Chemische Berichte, 1929, vol. 62, p. 579摘要:DOI:
-
作为产物:描述:参考文献:名称:Tarzia; Panzone; Leali, Farmaco, Edizione Scientifica, 1984, vol. 39, # 6, p. 538 - 558摘要:DOI:
文献信息
-
一种合成磺酰基酮酰胺的新方法
-
Direct Route to 1,3-Diketones by Palladium-Catalyzed Carbonylative Coupling of Aryl Halides with Acetylacetone作者:Signe Korsager、Dennis U. Nielsen、Rolf H. Taaning、Anders T. Lindhardt、Troels SkrydstrupDOI:10.1002/chem.201303872日期:2013.12.23Man up your magnesium! By employing a MgCl2/Et3N system, aryl diketones can be generated from the Pd‐catalyzed carbonylative α‐arylation of acetylacetone with aryl bromides (see scheme). The method is ideal for the introduction of carbon isotopes into more complex structures, since only stoichiometric amounts of carbon monoxide are employed.
-
Palladium catalyzed addition of arylboronic acid or indole to nitriles: synthesis of aryl ketones作者:Tuluma Das、Amarnath Chakraborty、Amitabha SarkarDOI:10.1016/j.tetlet.2014.11.009日期:2014.12Aryl ketones can be synthesized conveniently by a palladium catalyzed addition of arylboronic acid to nitriles in aqueous triflic acid. This catalytic system was extended to the addition of unprotected indoles to nitriles under a slightly modified condition to produce 3-acyl indoles in good yields.
-
Pd-catalyzed amidation of 1,3-diketones with CO and azides <i>via</i> a nitrene intermediate作者:Zheng-Yang Gu、Jie Chen、Ji-Bao XiaDOI:10.1039/d0cc04565a日期:——carbon monoxide and organic azides. This reaction provides a step-economic approach to produce β-ketoamides from readily available compounds under mild ligand-, oxidant-, and base-free conditions. The mechanistic studies showed that the reaction occurred through an in situ generated isocyanate intermediate.
-
[EN] NEW CRTH2 ANTAGONISTS<br/>[FR] NOUVEAUX ANTAGONISTES DE CRTH2申请人:ALMIRALL SA公开号:WO2013010880A1公开(公告)日:2013-01-24The present invention relates to compounds of formula (I), to the process for preparing such compounds and to their use in the treatment of a pathological condition or disease susceptible to amelioration by CRTh2 antagonist activity.本发明涉及式(I)的化合物,以及制备这种化合物的方法,以及它们在治疗病理状况或疾病中的应用,这些病理状况或疾病容易通过CRTh2拮抗活性得到改善。
表征谱图
-
氢谱1HNMR
-
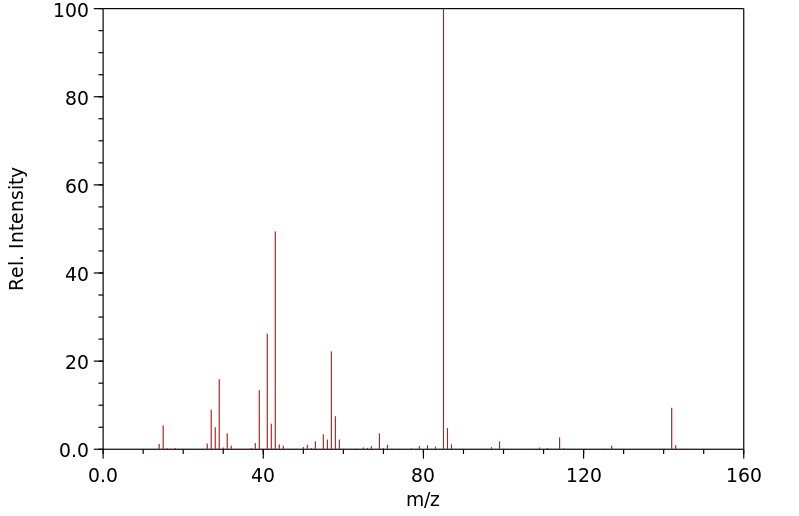
质谱MS
-
碳谱13CNMR
-
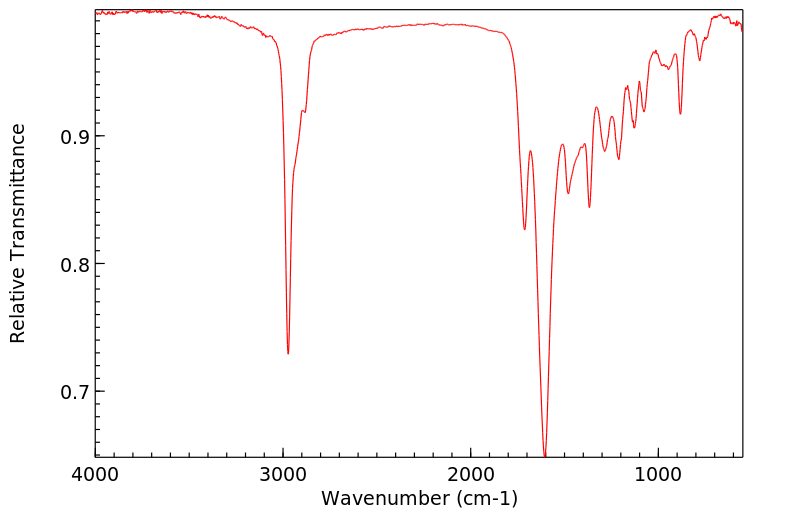
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷