1-氨基-4-溴-2-甲基蒽醌 | 81-50-5
中文名称
1-氨基-4-溴-2-甲基蒽醌
中文别名
1-氨基-4-溴基-2-甲基蒽醌;1-氨基-4-溴-2-甲基蒽酮;1-氨基-4-溴-2-甲基蒽并醌
英文名称
1-amino-2-methyl-4-bromoanthraquinone
英文别名
1-amino-4-bromo-2-methyl anthraquinone;1-Amino-4-bromo-2-methylanthraquinone;1-amino-4-bromo-2-methylanthracene-9,10-dione
CAS
81-50-5
化学式
C15H10BrNO2
mdl
MFCD00001221
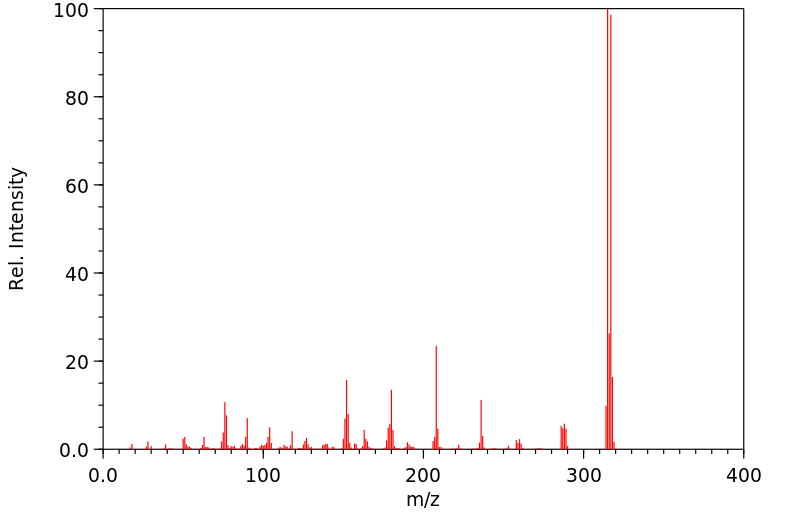
分子量
316.154
InChiKey
VIQMJMDPUIBXQO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:245 °C (dec.)(lit.)
-
稳定性/保质期:
与强氧化剂和强酸反应。
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:19
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.066
-
拓扑面积:60.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2922399090
-
储存条件:密封保存,存放在阴凉干燥的地方。
SDS
| Name: | 1-Amino-4-bromo-2-methylanthraquinone 95% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 81-50-5 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 81-50-5 | 1-Amino-4-bromo-2-methylanthraquinone | 95% | 201-355-6 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 81-50-5: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: orange to red
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 245 - 252 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H10BrNO2
Molecular Weight: 316.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 81-50-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Amino-4-bromo-2-methylanthraquinone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 81-50-5: No information available.
Canada
CAS# 81-50-5 is listed on Canada's NDSL List.
CAS# 81-50-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 81-50-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-Acetamino-2-methyl-4-brom-anthrachinon 101575-99-9 C17H12BrNO3 358.191 分散橙11 disperse orange 11 82-28-0 C15H11NO2 237.258 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Benzeneacetamide, N-(4-bromo-9,10-dihydro-2-methyl-9,10-dioxo-1-anthracenyl)- 67499-51-8 C23H16BrNO3 434.289 —— 1-bromo-3-methyl-9,10-anthraquinone 2952-23-0 C15H9BrO2 301.139 1-氨基-3-甲基蒽-9,10-二酮 1-amino-3-methyl-9,10-anthraquinone 10146-51-7 C15H11NO2 237.258 —— 1,4-diamino-2-methyl-anthraquinone 3225-95-4 C15H12N2O2 252.272 —— 1-bromo-3-dibromomethyl-anthraquinone 681464-64-2 C15H7Br3O2 458.931 —— 1-Amino-2-methyl-4-methylamino-anthrachinon 93013-66-2 C16H14N2O2 266.299 —— 1-Amino-2-methyl-4-(m-tolylamino)anthracene-9,10-dione 733748-40-8 C22H18N2O2 342.397 —— 1-amino-4-anilino-2-methyl-anthraquinone 75313-54-1 C21H16N2O2 328.37 1-氨基-2-甲基-4-[(4-甲基苯基)氨基]蒽-9,10-二酮 1-amino-2-methyl-4-p-toluidino-anthraquinone 116-77-8 C22H18N2O2 342.397 —— 1-amino-4-[(2-hydroxyethyl)amino]-2-methylanthracene-9,10-dione 3225-94-3 C17H16N2O3 296.326 —— 1-amino-4-hydroxy-2-methyl-anthraquinone 3225-96-5 C15H11NO3 253.257 —— 1-amino-4-{[3-(dimethylamino)propyl]amino}-2-methylanthra-9,10-quinone 501075-77-0 C20H23N3O2 337.422 —— 1-amino-2-methyl-4-(4-tert.-butylphenylmercapto)-anthraquinone 94176-30-4 C25H23NO2S 401.529 —— 5-[(4-Amino-3-methyl-9,10-dioxoanthracen-1-yl)amino]-2-anilinobenzenesulfonic acid 1052088-32-0 C27H21N3O5S 499.547 - 1
- 2
反应信息
-
作为反应物:描述:1-氨基-4-溴-2-甲基蒽醌 在 硫酸 、 potassium acetate 、 copper diacetate 作用下, 生成 N-(4-amino-3-methyl-9,10-dioxo-9,10-dihydro-[1]anthryl)-sulfanilic acid参考文献:名称:410.烷基对蒽醌和荧光素染料性能的影响摘要:DOI:10.1039/jr9360001838
-
作为产物:参考文献:名称:基于蒽醌衍生物接枝到O-羧甲基壳聚糖 上的新型高分子染料的合成和颜色特性摘要:通过Ullmann缩合将溴化蒽醌衍生物接枝到O-羧甲基壳聚糖上,制备了四种聚合染料。通过傅里叶变换红外光谱(FT-IR),元素分析和差示扫描量热法(DSC)对制备的高分子染料的化学结构进行了测定,结果表明蒽醌衍生物已成功接枝到O-羧甲基壳聚糖上。四种聚合染料的接枝度分别为0.66、1.14、0.93和1.01 mmol g -1分别。使用数码照片和UV-Vis吸收光谱确定并比较了溴代蒽醌衍生物和制备的聚合物染料的色泽性能,发现蒽醌衍生物中取代基的电子性质和平面度明显影响其吸附波长。制备的聚合物染料。另外,具有给电子基团和较高平面度的聚合物染料显示更长的吸收波长和更深的颜色。测试了所制备的聚合染料的细胞毒性,其在人肝细胞系LO2上的IC 50值分别为7.666、8.557、8.186和8.934 g L -1,表明所制备的聚合染料的细胞毒性较低。DOI:10.1039/c7ra04024e
文献信息
-
Ullmann reactions of 1-amino-4-bromoanthraquinones bearing various 2-substituents furnishing novel dyes作者:Enas M. Malik、Mahmoud Rashed、Lukas Wingen、Younis Baqi、Christa E. MüllerDOI:10.1016/j.dyepig.2016.03.023日期:2016.8Novel 1-amino-4-(ar)alkylaminoanthraquinone derivatives bearing different substituents (Br, CH2OH, CN, or CH3) at the 2-position of the anthraquinone scaffold were synthesized by copper-catalyzed Ullmann condensation. The 2-substituted 1-amino-4-bromoanthraquinone derivatives (bromaminic acid analogues) were reacted with a variety of alkyl-, aryl-, and aralkylamines. Different reaction conditions were
-
Synthesis and In Silico Study of 4-Substituted 1-Aminoanthraquinones作者:V. I. Shupeniuk、N. Amaladoss、T. N. Taras、O. P. Sabadakh、N. P. MatkivskyiDOI:10.1134/s1070428021040126日期:2021.44-substituted 1-amino-9,10-anthraquinones containing a primary amino group were synthesized by nucleophilic substitution of bromine in 1-amino-4-bromo-9,10-anthraquinones. 1-Amino-4-[2-(hydroxyethyl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid containing a biogenic amine fragment (2-aminoethanol) was converted into the corresponding 1-triazenyl derivatives. The structure of the synthesized摘要 通过在1-氨基-4-溴-9,10-蒽醌中溴的亲核取代合成了八个含伯氨基的新的4-取代的1-氨基-9,10-蒽醌。含有生物胺片段(2-氨基乙醇)的1-氨基-4- [2-(羟乙基)氨基] -9,10-二氧代-9,10-二氢蒽-2-磺酸被转化为相应的1-三氮烯衍生物。根据LC / MS和13 C和1 H NMR数据确定合成的化合物的结构,并通过计算机估计其药物相似性。用DIGEP-Pred分析了具有良好药物相似性评分的化合物,使用STRING模拟了它们与蛋白质的可能相互作用,并使用《京都议定书》(Kyoto Encyclopedia of Genes and Genomes)解释了它们的生物学活性。
-
Water-soluble amine-linked polymeric colorants申请人:Dynapol公开号:US04051138A1公开(公告)日:1977-09-27Polymeric colorants comprising a hydrocarbon backbone and attached directly thereto through amine linkages, at least one water-solubilizing group and at least one optically chromophoric group are disclosed. In a preferred embodiment, the backbone is a saturated aliphatic hydrocarbon, the chromophore is an anthraquinone chromophore and the solubilizing group is a sulfonate or sulfamate residue.
-
Development of Potent and Selective Antagonists for the UTP-Activated P2Y<sub>4</sub> Receptor作者:Muhammad Rafehi、Enas M. Malik、Alexander Neumann、Aliaa Abdelrahman、Theodor Hanck、Vigneshwaran Namasivayam、Christa E. Müller、Younis BaqiDOI:10.1021/acs.jmedchem.7b00030日期:2017.4.13nM, selectivity versus other P2Y receptor subtypes, and is thought to act as an allosteric antagonist. A receptor homology model was built and docking studies were performed to analyze ligand–receptor interactions. Compound 64 (PSB-1699, sodium 1-amino-4-[4-(3-pyridin-3-ylmethylthio)phenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate) represents the most selective P2Y4 receptor antagonist knownP2Y 4是由尿苷5'-三磷酸(UTP)激活的Gq蛋白偶联受体,在体内,例如在肠,心脏和脑中广泛表达。到目前为止,尚未描述选择性P2Y 4受体拮抗剂。因此,我们开发和优化了基于蒽醌支架的P2Y 4受体拮抗剂。通过基于荧光的测定法评估效价,该测定法测量了稳定转染了人P2Y 4受体的1321N1星形细胞瘤细胞中UTP诱导的细胞内钙释放的抑制作用。本系列中最有效的化合物,钠1-氨基-4- [4-(2,4-二甲基苯硫基)苯基氨基] -9,10-二氧代-9,10-二氢蒽-2-磺酸盐(PSB-16133,61)表现出233 nM的IC 50值,相对于其他P2Y受体亚型具有选择性,并被认为是一种变构拮抗剂。建立了受体同源性模型,并进行了对接研究以分析配体-受体的相互作用。化合物64(PSB-1699,1-氨基-4- [4-(3-吡啶-3-基甲硫基)苯基氨基] -9,10-二氧代-9,10-二氢蒽-2-磺
-
METHODS OF DESIGNING, PREPARING, AND USING NOVEL PROTONOPHORES申请人:Martineau Louis C.公开号:US20140135359A1公开(公告)日:2014-05-15The present invention provides a computer-assisted method of generating a protonophore requiring the use of a computer including a processor. The method includes: designing the protonophore, calculating, using the processor, an estimated protonophoric activity; producing the protonophore if the estimated protonophoric activity corresponds to an U 50 of about 20 μM or less; and determining the uncoupling activity of the protonophore. The present invention also provides novel protonophores that meet the above requirement and their methods of use.本发明提供了一种利用计算机辅助的方法来生成需要使用处理器的质子载体。该方法包括:设计质子载体,使用处理器计算估计的质子载体活性;如果估计的质子载体活性对应于大约20微米或更少的U50,则生产质子载体;并确定质子载体的解耦活性。本发明还提供了符合上述要求的新型质子载体及其使用方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
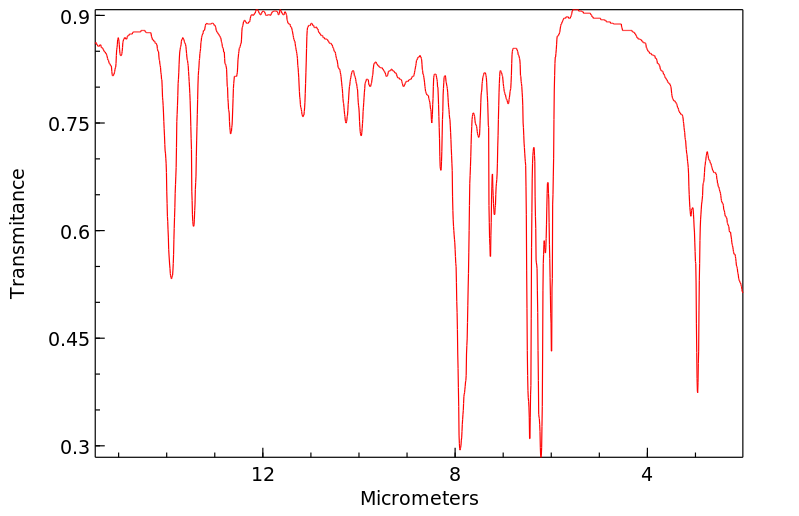
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62