3,4-二甲氧基-5-羟基苯甲酸 | 1916-08-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:197-198°C
-
沸点:374.2±42.0 °C(Predicted)
-
密度:1.335±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,没有已知的危险反应。请避免接触氧化剂。
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:76
-
氢给体数:2
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2918990090
-
储存条件:密封,在2°C至-8°C的条件下保存。
SDS
制备方法与用途
3,4-二甲氧基-5-羟基苯甲酸是一种有用的研究化合物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲氧基-5-羟基苯甲酸甲酯 3-hydroxy-4,5-dimethoxybenzoate 83011-43-2 C10H12O5 212.202 3,4-二甲氧基-5-羟基苯甲醛 3-hydroxy-4,5-dimethoxybenzaldehyde 29865-90-5 C9H10O4 182.176 没食子酸 3,4,5-trihydroxybenzoic acid 149-91-7 C7H6O5 170.122 3-苯甲酰氧基-4,5-二羟基-苯甲酸 3-benzoyloxy-4,5-dihydroxy-benzoic acid 29970-31-8 C14H10O6 274.23 香草醛 vanillin 121-33-5 C8H8O3 152.15 3-溴-4,5-二甲氧基苯甲酸 3-bromo-4,5-dimethoxybenzoic acid 20731-48-0 C9H9BrO4 261.072 3-溴-4,5-二甲氧基苯甲醛 5-bromoveratralaldehyde 6948-30-7 C9H9BrO3 245.073 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲氧基-5-羟基苯甲酸甲酯 3-hydroxy-4,5-dimethoxybenzoate 83011-43-2 C10H12O5 212.202 3,4,5-三甲氧基苯甲酸甲酯 3,4,5-trimethoxybenzoic acid methyl ester 1916-07-0 C11H14O5 226.229 —— 3-ethoxy-4,5-dimethoxy-benzoic acid 214849-37-3 C11H14O5 226.229 3,4-二甲氧基-5-羟基苯甲醛 3-hydroxy-4,5-dimethoxybenzaldehyde 29865-90-5 C9H10O4 182.176 —— 3-acetoxy-4,5-dimethoxybenzoic acid 5444-05-3 C11H12O6 240.213 —— methyl 3-benzyloxy-4,5-dimethoxybenzoate 26409-24-5 C17H18O5 302.327 —— 3,4-dimethoxy-5-methoxycarbonyloxy-benzoic acid 854872-79-0 C11H12O7 256.212 —— 3-benzyloxy-4,5-dimethoxybenzyl alcohol 7632-62-4 C16H18O4 274.317 —— 5-hydroxy-3,4-dimethoxyphthaldehydic acid 82448-57-5 C10H10O6 226.186 —— 3-[2-(2,4-dichlorophenyl)-ethoxy]-4,5-dimethoxy-benzoic acid 438571-01-8 C17H16Cl2O5 371.217 —— 5-hydroxy-3,4-dimethoxyphthalic acid 81892-86-6 C10H10O7 242.185 —— 2-bromo-4,5-dimethoxy-3-hydroxybenzoic acid methyl ester 236392-94-2 C10H11BrO5 291.098 —— methyl 3,4-dimethoxy-5-[(methylsulfonyl)oxy]benzoate 1344112-25-9 C11H14O7S 290.294 —— 4,5-dimethoxy-3-hydroxy-2-vinylbenzoic acid methyl ester 236392-95-3 C12H14O5 238.24 —— Iridonitril, 3-Hydroxy-4,5-dimethyl-benzylcyanid 408335-71-7 C10H11NO3 193.202 - 1
- 2
反应信息
-
作为反应物:描述:3,4-二甲氧基-5-羟基苯甲酸 在 吡啶 、 lithium aluminium tetrahydride 、 氯化亚砜 、 硫酸 、 potassium carbonate 作用下, 生成 3-benzyloxy-4,5-dimethoxybenzyl chloride参考文献:名称:异黄酮的合成。第五部分Irigenin和Tectorigenin摘要:合成异黄酮的一般乙氧基化方法已应用于合成irigenin(VII)和tectorigenin(XVI)。该合成途径也产生了异构体,ψ -irigenin(XI)和ψ -tectorigenin(XX)。讨论了有关某些乙氧基化反应中产物比例以及5,6,7-和5,7,8-三取代异黄酮的相对热力学稳定性的信息。DOI:10.1039/j39700001219
-
作为产物:描述:参考文献:名称:Shriner; McCutchan, Journal of the American Chemical Society, 1929, vol. 51, p. 2194摘要:DOI:
文献信息
-
Ruthenium-Catalyzed Hydroarylation and One-Pot Twofold Unsymmetrical C−H Functionalization of Arenes作者:Koushik Ghosh、Raja K. Rit、E. Ramesh、Akhila K. SahooDOI:10.1002/anie.201600649日期:2016.6.27excellent yields. A one‐pot, unsymmetrical, twofold C−H functionalization involving intramolecular C−C and intermolecular C−C/C−N bond formations is successfully demonstrated by using a single set of catalytic reaction conditions, which is unprecedented thus far. A novel isoquinolone‐bearing dihydrobenzofuran is constructed through an unsymmetrical twofold C−H functionalization.
-
New thiadiazoles and their use as phosphodiesterase-7 inhibitors申请人:WARNER-LAMBERT COMPANY公开号:EP1193261A1公开(公告)日:2002-04-03The invention provides 1,3,4-thiadiazoles having the following formula (I): in which, R1 is alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, cycloalkenyl, aryl, heteroaryl or a polycyclic group, optionally substituted, R2 is alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, cycloalkenyl or aryl optionally substituted, R3 is X2-R'3, in which X2 is a binding group and R'3 is cycloalkyl, heterocycloalkyl, cycloalkenyl, aryl, heteroaryl, or a polycyclic group; optionally substituted, or their pharmaceutically acceptable derivatives, the process for their preparation and their use for the manufacture of a medicament for the treatment of disorders for which a treatment by a PDE7 inhibitor is relevant.
-
[EN] LEVORPHANOL PRODRUGS AND PROCESSES FOR MAKING AND USING THEM<br/>[FR] PROMÉDICAMENTS DE LEVORANOL ET LEURS PROCÉDÉS DE FABRICATION ET D'UTILISATION申请人:KEMPHARM INC公开号:WO2018191472A1公开(公告)日:2018-10-18The presently described technology provides compositions of one or more of oxoacids, polyethylene glycols, and vitamin compounds chemically conjugated to levorphanol ((-)-17-methylmorphinan-3-ol) to form novel prodrugs and compositions of levorphanol.
-
New oxybenzamide derivatives useful for inhibiting factor Xa or VIIa申请人:——公开号:US20020198195A1公开(公告)日:2002-12-26The present invention relates to compounds comprising the following formula: R 0 —Q—X—Q′—W—U—V—G—M (I) These compounds are useful as pharmacologically active compounds. They exhibit an antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders such as thromboembolic diseases or restenoses. These compounds are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and/or factor VIIa (FVIIa), and can generally be used to treat, prevent, or cure conditions in which an undesired activity of factor Xa and/or factor VIIa is present, or where inhibition of factor Xa and/or factor VIIa is intended. The invention further relates to processes for the preparation of these compounds, methods of their use (e.g., as active ingredients in pharmaceuticals), and pharmaceutical preparations comprising them.本发明涉及包含以下公式的化合物: R 0 —Q—X—Q′—W—U—V—G—M (I) 这些化合物作为药物活性化合物是有用的。它们展现出抗血栓作用,并适用于例如治疗和预防心血管疾病,如血栓栓塞性疾病或再狭窄。这些化合物是血液凝固酶因子Xa(FXa)和/或因子VIIa(FVIIa)的可逆性抑制剂,通常可用于治疗、预防或治愈存在因子Xa和/或因子VIIa不期望活性的情况,或旨在抑制因子Xa和/或因子VIIa的情况。本发明还涉及制备这些化合物的方法、它们的使用方法(例如,作为药品中的活性成分)以及包含它们的药物制剂。
-
Isolation, structure, and synthesis of combretastatin A-2, A-3, and B-2作者:George R. Pettit、Sheo Bux SinghDOI:10.1139/v87-399日期:1987.10.1
Further investigation of the South African tree Combretumcaffrum (Combretaceae) for murine P388 lymphocytic leukemia (PS) cell-growth inhibitory substances has led to discovery of three new active constituents designated combretastatins A-2 (5a, PS ED50 0.027 μg/mL), A-3 (5b, PS ED50 0.026 μg/mL), and B-2 (3b, PS ED50 0.32 μg/mL). Both combretastatins A-2 and A-3 were found to markedly inhibit tubulin polymerization. The structure of each combretastatin was firmly established by a combination of high resolution (400 MHz) 1H and 13C nuclear magnetic resonance and mass spectral analyses followed by total syntheses. The conversion of methyl gallate (7b) to combretastatin A-2 via intermediates 7c → 7d → 7e → 7a and 6a → 5a illustrates the practical synthetic route utilized for obtaining these substances. The Wittig reaction employed as the penultimate step in obtaining combretastatins A-3, afforded predominantly the natural Z isomer.
对南非树木Combretum caffrum(锥花科)进行进一步研究,以寻找对小鼠P388淋巴细胞白血病(PS)细胞生长具有抑制作用的物质,发现了三种新的活性成分,分别命名为combretastatin A-2(5a,PS ED50 0.027 μg/mL)、A-3(5b,PS ED50 0.026 μg/mL)和B-2(3b,PS ED50 0.32 μg/mL)。发现combretastatin A-2和A-3均明显抑制微管聚合。通过高分辨率(400 MHz)的1H和13C核磁共振和质谱分析以及总合成,确定了每种combretastatin的结构。通过从甲基没食子酸酯(7b)经过中间体7c→7d→7e→7a和6a→5a的转化,说明了用于获得这些物质的实用合成路线。在获得combretastatin A-3的倒数第二步中采用的Wittig反应主要产生了天然的Z异构体。
表征谱图
-
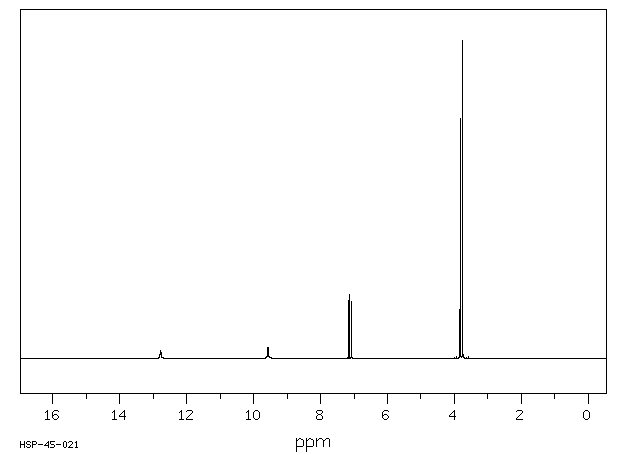
氢谱1HNMR
-
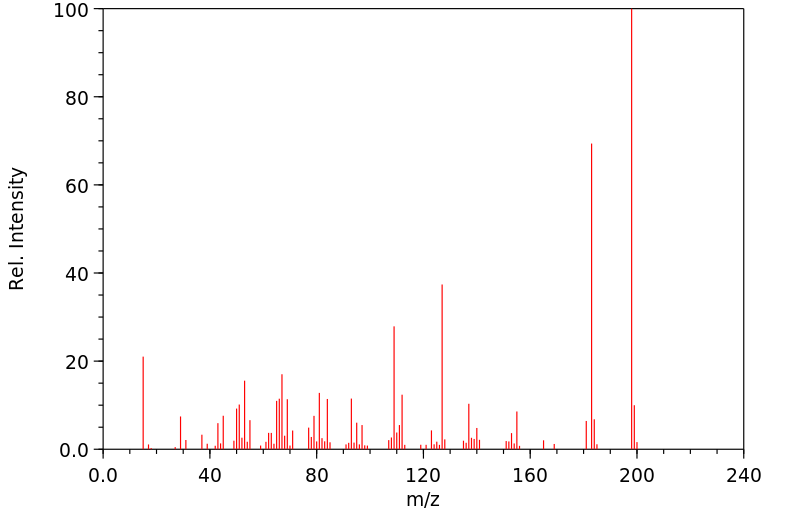
质谱MS
-
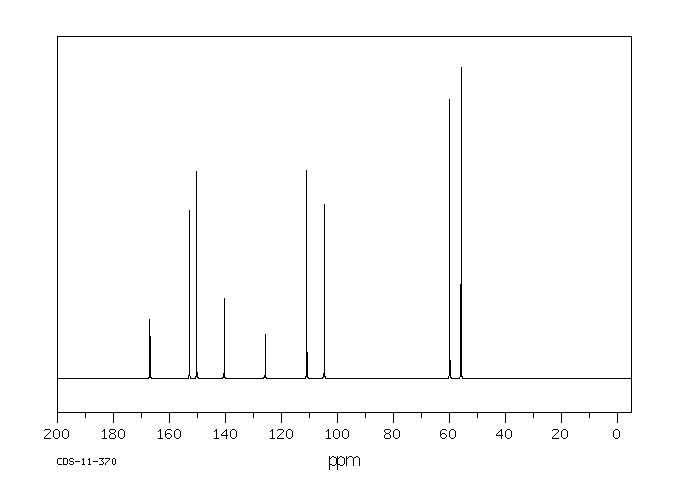
碳谱13CNMR
-
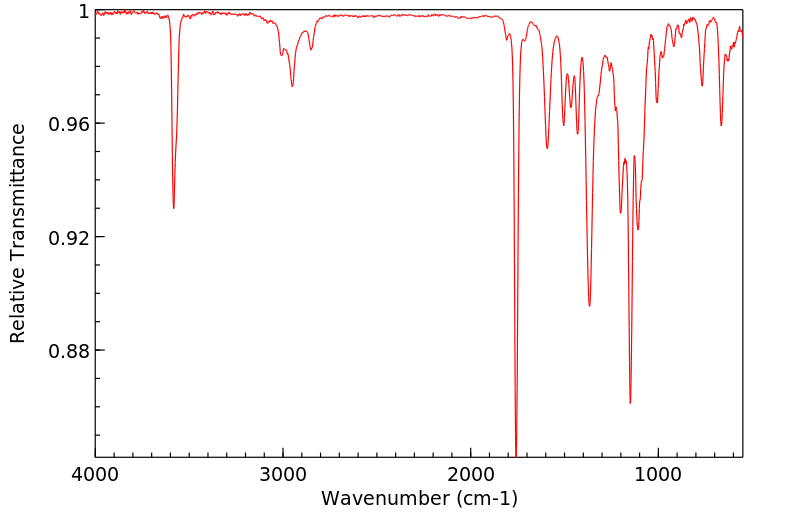
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息