十八烷基二甲基叔胺 | 124-28-7
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:23 °C
-
沸点:347 °C
-
密度:0.8
-
闪点:155 °C
-
溶解度:氯仿(微溶)、己烷(微溶)
-
LogP:1.3-5.1 at 20℃ and pH7.1-8.8
-
表面张力:32.2mN/m at 1g/L and 25℃
-
解离常数:9.78 at 25℃
-
物理描述:N,n-dimethyl-1-octadecanamine appears as a clear yellow liquid with a fishlike odor. Insoluble in water and less dense than water. Hence floats on water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation or skin absorption. Used to make other chemicals.
-
保留指数:2096
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):9.1
-
重原子数:21
-
可旋转键数:17
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R22,R34
-
海关编码:2921199090
-
危险品运输编号:2735
-
RTECS号:RG4200000
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P233,P260,P261,P264,P271,P273,P280,P301+P330+P331,P303+P361+P353,P304,P304+P340,P305+P351+P338,P310,P312,P321,P340,P363,P391,P403,P403+P233,P405,P501
-
危险性描述:H314,H336,H400,H410
-
储存条件:塑料桶包装,每桶重50千克。应贮存于阴凉、通风的地方,并远离火种和热源。 在长途运输时,外用木条箱保护,并在桶底衬垫稻草以增加安全性。
SDS
| Name: | N N-Dimethyloctadecylamine (Techn) 89% (GC) Material Safety Data Sheet |
| Synonym: | Dimethylstearamine; Dymanthine |
| CAS: | 124-28-7 |
Synonym:Dimethylstearamine; Dymanthine
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 124-28-7 | N,N-Dimethyloctadecylamine | 89 | 204-694-8 |
Risk Phrases: 10 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Causes burns.Corrosive.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause cyanosis of the extremities. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. The toxicological properties of this substance have not been fully investigated. Ingestion of large amounts may cause CNS depression. May cause systemic effects.
Inhalation:
Causes chemical burns to the respiratory tract. The toxicological properties of this substance have not been fully investigated.
Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation. May cause systemic effects. May cause burning sensation in the chest.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Water may be ineffective. Material is lighter than water and a fire may be spread by the use of water. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame.
Avoid ingestion and inhalation. Discard contaminated shoes. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 124-28-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: amine-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 23 deg C
Autoignition Temperature: Not available.
Flash Point: 44 deg C ( 111.20 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8000g/cm3
Molecular Formula: C20H43N
Molecular Weight: 297.57
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 124-28-7: RG4200000 LD50/LC50:
CAS# 124-28-7: Draize test, rabbit, eye: 20 mg/24H Moderate; Draize test, rabbit, skin: 20 mg/24H Moderate.
Carcinogenicity:
N,N-Dimethyloctadecylamine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Amines, liquid, corrosive, n.o.s.*
Hazard Class: 8 (3)
UN Number: 2735
Packing Group: III
IMO
Shipping Name: Amines, liquid, corrosive, n.o.s.
Hazard Class: 8 (3)
UN Number: 2735
Packing Group: III
RID/ADR
Shipping Name: CORROSIVE LIQUID, FLAMMABLE, N.O.S.
Hazard Class: 8
UN Number: 2920
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 10 Flammable.
R 34 Causes burns.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 25 Avoid contact with eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 124-28-7: 2
Canada
CAS# 124-28-7 is listed on Canada's DSL List.
CAS# 124-28-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 124-28-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
这是一种浅棕色粘稠液体,在20℃时则为浅草黄色软质固体。它易溶于醇类溶剂,而不溶于水。
用途该物质是季铵盐型阳离子表面活性剂的重要有机合成中间体,可用于生成不同的季铵盐阳离子。这些反应可以与环氧乙烷、硫酸二甲酯、硫酸二乙酯、氯甲烷或氯苄进行。由此获得的产物可应用于织物柔软、抗静电剂以及改善头发梳理性等产品中。此外,它还可用以生产驱虫剂。N,N-二甲基十八胺经过与环氧乙烷和硝酸反应后,可以制得十八烷基二甲基羟乙基季铵硝酸盐(即抗静电剂SN)。
用途 生产方法N,N-二甲基十八胺可通过将十八胺与甲醛和甲酸缩合获得。具体步骤是首先在乙醇介质中搅拌均匀十八胺,随后在50-60℃下加入甲酸,搅拌数分钟后,在60-65℃下加入甲醛,升温至80-83℃并回流保温2小时后用液碱中和至pH值大于10,静置分层去水后再进行减压蒸馏去除乙醇。冷却后即可得到N,N-二甲基十八胺。
原料消耗定额如下:每吨产品需要工业品十八胺917千克、95%乙醇417千克、甲酸510千克和37%甲醛546千克。此外,还有直接由高级醇与二甲胺合成的方法。该方法是在存在催化剂的情况下,在180-220℃的液相条件下将十八醇与二甲胺进行催化胺化反应,并脱除一分子水后得到粗叔胺,再通过减压蒸馏获得高纯度N,N-二甲基十八胺。每吨产品需要消耗十八醇971千克和二甲胺170千克。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 双十八烷基胺 Dioctadecylamine 112-99-2 C36H75N 521.998 十八胺 1-aminooctadecane 124-30-1 C18H39N 269.514 N n-二甲基-9-十八酰胺 N,N-dimethyloctadecanamide 3886-90-6 C20H41NO 311.552 N,N-二甲基十二酰胺 N,N-dimethyldodecanamide 3007-53-2 C14H29NO 227.39 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基十八烷基-N-氧化胺 N,N-dimethyloctadecylamine-N-oxide 2571-88-2 C20H43NO 313.568
反应信息
-
作为反应物:描述:参考文献:名称:新型的低粘度,绿色和两亲性N-氧化物/苯乙酸基深共熔溶剂摘要:通过将苯乙酸(一种存在于蜂蜜中的天然分子)与胺N-氧化物(自然界中易于生物降解的分子)混合,制得了四种新型的深共晶溶剂(DES)。这些N-氧化物中的三个是两亲的。新型DES的凝固点非常低(从-34°C到20°C),并且粘度非常低,远低于迄今为止文献中最常用和使用的DES(氯化胆碱/尿素混合物)。电导率值较低,离子性分析表明这些DES为“超离子性”。它们的极性足够高,可以与其他常用溶剂或离子液体相比。DOI:10.1016/j.molliq.2017.05.084
-
作为产物:描述:N n-二甲基-9-十八酰胺 在 sponge copper catalyst: 73.4percent Cu, 0.5percent Mg, 5.7percent Fe, 0.9percent Al 氢气 作用下, 以 水 为溶剂, 250.0 ℃ 、1.5 MPa 条件下, 以81.7%的产率得到十八烷基二甲基叔胺参考文献:名称:PROCESS FOR PRODUCING NITROGEN-CONTAINING COMPOUNDS摘要:本发明涉及一种在存在含有海绵铜催化剂的催化剂的情况下,通过还原酰胺化合物来生产三级胺的方法,所述海绵铜催化剂是通过浸出含有铜和铝的合金颗粒并干燥浸出的合金颗粒获得的。本发明提供了一种在无溶剂适度条件下将脂肪酸酰胺经过加氢还原处理以高产率产生含有少量副产物的高纯度脂肪族三级胺的方法。公开号:US20100179349A1
-
作为试剂:参考文献:名称:[EN] PROCESS FOR THE PREPARATION OF ANTICONVULSANT DERIVATIVES OF TOPIRAMATE
[FR] PROCEDE DE PREPARATION DE DERIVES DE TOPIRAMATE ANTICONVULSIFS摘要:本发明涉及一种制备一步法的果糖吡喃磺酰胺衍生物的方法,其通式为(I),其中X、R1、R3、R4、R5和R6如规范中所述。公开号:WO2004078769A1
文献信息
-
USE OF NITROGEN COMPOUNDS QUATERNISED WITH ALKYLENE OXIDE AND HYDROCARBYL-SUBSTITUTED POLYCARBOXYLIC ACID AS ADDITIVES IN FUELS AND LUBRICANTS申请人:BASF SE公开号:US20160130514A1公开(公告)日:2016-05-12The invention relates to the use of quaternized nitrogen compounds as a fuel and lubricant additive or kerosene additive, such as in particular as a detergent additive, for decreasing or preventing deposits in the injection systems of direct-injection diesel engines, in particular in common rail injection systems, for decreasing the fuel consumption of direct-injection diesel engines, in particular of diesel engines having common rail injection systems, and for minimizing the power loss in direct-injection diesel engines, in particular in diesel engines having common rail injection systems; the invention further relates to the use as an additive for petrol, in particular for operation of DISI engines.该发明涉及将季铵化氮化合物用作燃料和润滑剂添加剂或煤油添加剂,特别是作为清洁剂添加剂,用于减少或预防直喷柴油发动机的喷射系统中的沉积物,在特定是在共轨喷射系统中,用于降低直喷柴油发动机的燃料消耗,特别是具有共轨喷射系统的柴油发动机,并用于减少直喷柴油发动机的功率损失,特别是在具有共轨喷射系统的柴油发动机中;该发明还涉及将其用作汽油添加剂,特别是用于DISI发动机的运行。
-
Synthesis of 4-ketocyclopentene and cyclopentadiene compounds申请人:——公开号:US20020002308A1公开(公告)日:2002-01-03A process for forming 4-ketocyclopentene and substituted 4-ketocyclopentene compounds starting from the corresponding 1-carbohydrocarbyloxy-2-keto-4-hydroxy-5-cyclopentene by reduction followed by decarboxylation using zinc dichloride or zinc dibromide. The ketone may thereafter be converted to a cyclopentadiene compound by reducing the ketone to form an alcohol, replacing the hydroxyl functionality of the alcohol under substitution conditions with a leaving group, and deprotonating the resulting product under base induced elimination conditions to form the cyclopentadiene compound. Alternatively functionalized cyclopentadienyl compounds can be produced without isolation of an unsubstituted cyclopentadienyl compound by combining the elimination and deprotonation steps with a replacement step in a single unit operation.
-
Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part作者:Bogumil Eugene Brycki、Adrianna Szulc、Iwona Kowalczyk、Anna Koziróg、Ewelina SobolewskaDOI:10.3390/molecules26195759日期:——
Due to their large possibility of the structure modification, alkylammonium gemini surfactants are a rapidly growing class of compounds. They exhibit significant surface, aggregation and antimicrobial properties. Due to the fact that, in order to achieve the desired utility effect, the minimal concentration of compounds are used, they are in line with the principle of greenolution (green evolution) in chemistry. In this study, we present innovative synthesis of the homologous series of gemini surfactants modified at the spacer by the ether group, i.e., 3-oxa-1,5-pentane-bis(N-alkyl-N,N-dimethylammonium bromides). The critical micelle concentrations were determined. The minimal inhibitory concentrations of the synthesized compounds were determined against bacteria Escherichia coli ATCC 10536 and Staphylococcus aureus ATCC 6538; yeast Candida albicans ATCC 10231; and molds Aspergillus niger ATCC 16401 and Penicillium chrysogenum ATCC 60739. We also investigated the relationship between antimicrobial activity and alkyl chain length or the nature of the spacer. The obtained results indicate that the synthesized compounds are effective microbicides with a broad spectrum of biocidal activity.
由于烷基铵双链表面活性剂具有结构修改的大可能性,它们是一类快速增长的化合物。它们表现出显著的表面、聚集和抗微生物特性。由于为了实现所需的效果,使用了化合物的最小浓度,它们符合化学中的绿色演化原则。在这项研究中,我们提出了一种创新的合成方法,合成了通过醚基修饰的同系列双链表面活性剂,即3-氧杂-1,5-戊烷-双(N-烷基-N,N-二甲基铵溴化物)。确定了临界胶束浓度。确定了合成化合物对大肠杆菌ATCC 10536和金黄色葡萄球菌ATCC 6538;酵母白念珠菌ATCC 10231;以及曼氏黑曲霉ATCC 16401和青霉菌ATCC 60739的最小抑菌浓度。我们还研究了抗微生物活性与烷基链长度或间隔物性质之间的关系。得到的结果表明,合成的化合物是具有广谱生物杀灭活性的有效微生物杀菌剂。 -
Effect of the alkyl chain length on micelle formation for bis(N-alkyl-N,N-dimethylethylammonium)ether dibromides作者:Bogumil Brycki、Adrianna Szulc、Hanna Koenig、Iwona Kowalczyk、Tomasz Pospieszny、Sara GórkaDOI:10.1016/j.crci.2019.04.002日期:2019.5Résumé Dimeric alkylammonium salts – gemini surfactants – due to their unusual very low critical micelle concentration and minimal inhibitory concentration are subject to intensive research as surface active and antimicrobial compounds. Thanks to the presence of two positively charged nitrogen atoms and a large molecular surface, gemini surfactants are also very efficient corrosion inhibitors. To strengthen the electrostatic adsorption of ammonium cations on a metal surface, which is a key parameter in the inhibition of corrosion, heteroatoms (O, S, N, or P) and π-electron systems can be introduced into the gemini surfactant structure to increase chemical adsorption. In this study, we investigated the relationship between the alkyl chain length and critical micelle concentration for gemini surfactants containing an oxygen atom in the spacer, that is, bis(N-alkyl-N,N-dimethylethylammonium)ether dibromides, for potential use as corrosion inhibitors.
-
CONTACT-KILLING, QAC FUNCTIONALIZED THERMOPLASTIC POLYURETHANE FOR CATHETER APPLICATIONS申请人:THE UNIVERSITY OF AKRON公开号:US20190106525A1公开(公告)日:2019-04-11In various embodiments, the present invention provides a functionalized thermoplastic polyurethane (TPU) containing bulk incorporated or surface-grafted quaternary ammonium compounds (QAC)s for contact-killing of a variety of microbes, where the QACs are on the surface of TPU to provide a sterile surface material that prevents bacteria commonly involved in device-associated infections (DAIs) from proliferating. The functionalized TPUs of the present invention can be formed into a wide variety of 3-dimensional shapes, such as catheters, medical tubing, laryngeal or tracheal stents, sutures, prosthetics, wound dressings, and/or a coating for medical devices and contains the residue of either a QAC containing diol monomer or an alkene functional diol monomer, which then allows the TPU to be functionalized with a QAC containing disulfide or free thiol compound, to form a quaternary ammonium functionalized thermoplastic polyurethane compound having antimicrobial properties for use in medical devices.
表征谱图
-
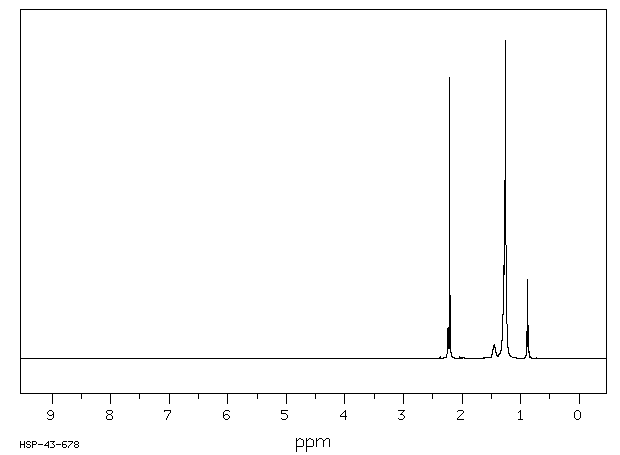
氢谱1HNMR
-
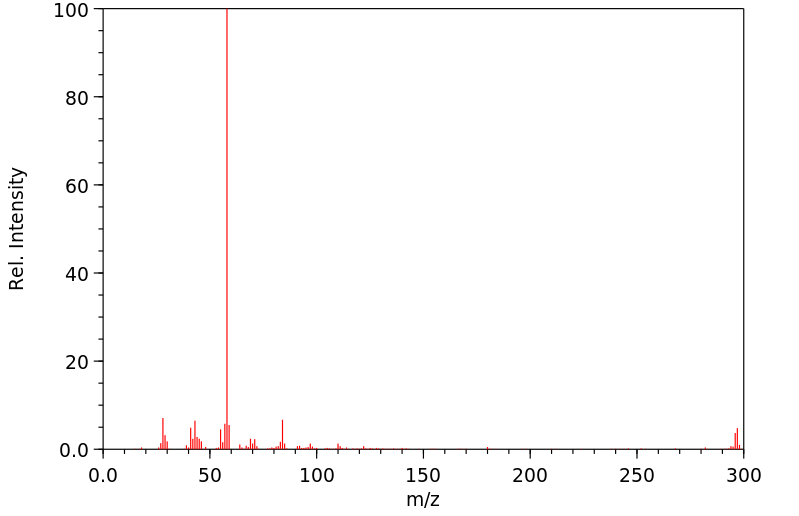
质谱MS
-
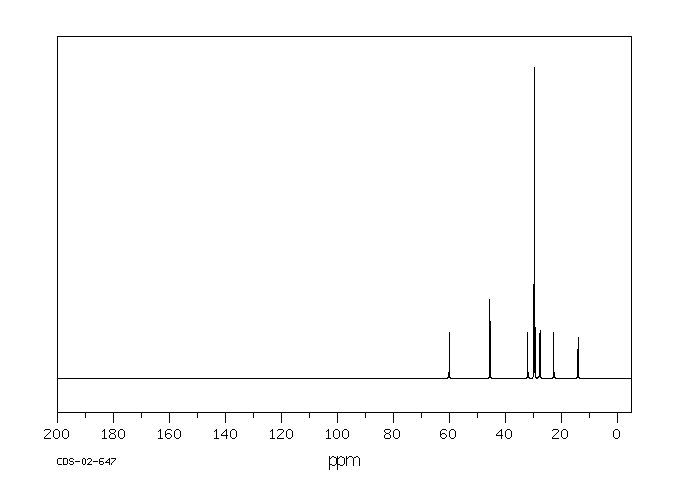
碳谱13CNMR
-
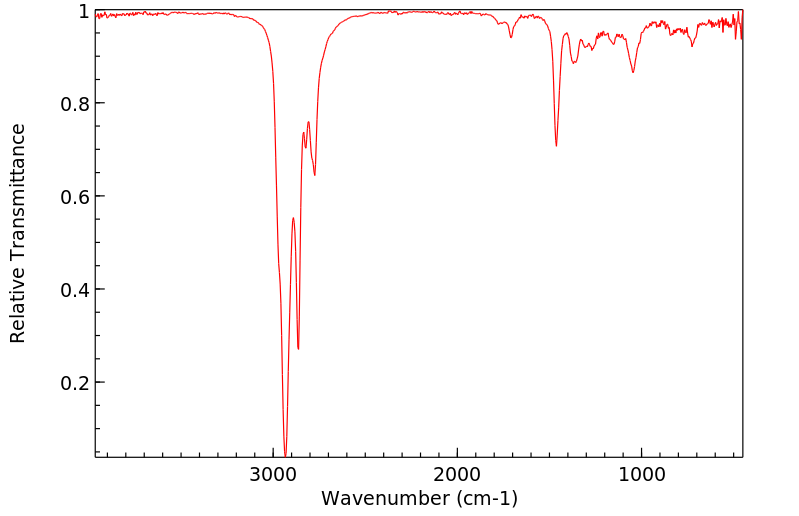
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息